Solid state form of enzalutamide, preparation method and use thereof
A technology of enzalutamide and amorphous state, which is applied in the field of medicinal chemical crystallization to achieve the effects of high solubility, good storage stability and low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
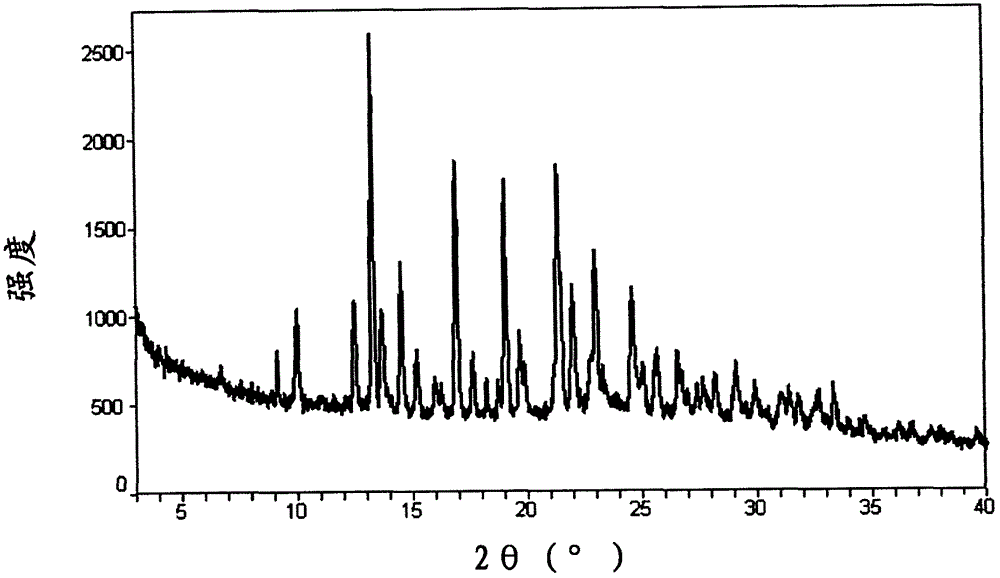
Image
Examples
Embodiment 1
[0112] Enzalutamide was synthesized according to the synthetic method of Patent Document US7709517 Example 56, specifically:
[0113] N-methyl-2-fluoro-4-(1,1-dimethyl-cyanomethyl)aminobenzamide (3g, 13mmol) and 4-isothiocyanato-2-trifluoromethylbenzene Formonitrile (5.8g, 26mmol) was added to 100mL DMF, heated to 100°C for 11 hours under microwave irradiation, cooled to 50°C, added 2000mL of methanol and 500mL of 1N hydrochloric acid, then heated to reflux for 1.5 hours, cooled to room temperature, poured into In 5000mL of cold water, extracted with 5000mL of ethyl acetate, the organic phase was dried by adding magnesium sulfate, filtered, the filtrate was concentrated, and the obtained residue was subjected to column chromatography (developing solvent: methylene chloride: acetone = 95: 5) to obtain 1.5 g of Enza Lutamine is a white solid, molar yield: 25%.
[0114] 1 H-NMR (300MHz, DMSO-d6): 1.61(s, 6H), 3.07(d, 3H, J=4.1Hz), 6.71(m, 1H), 7.15(dd, 1H, J=11.7Hz), 7.24 (dd,...
Embodiment 2
[0121] Take 100 mg of enzalutamide prepared in Example 1 and put it into a 30 mL single-necked flask, add 10 mL of dichloromethane, ultrasonicate at 40 KHz for 30 seconds to ensure that it is completely dissolved, then place it on a rotary evaporator, spin dry at 40 ° C, and remove the solvent The rate is about 15 ml / min, resulting in amorphous enzalutamide.
[0122] XRPD patterns such as Image 6 As shown, it appears to be amorphous without any sharp diffraction peaks. .
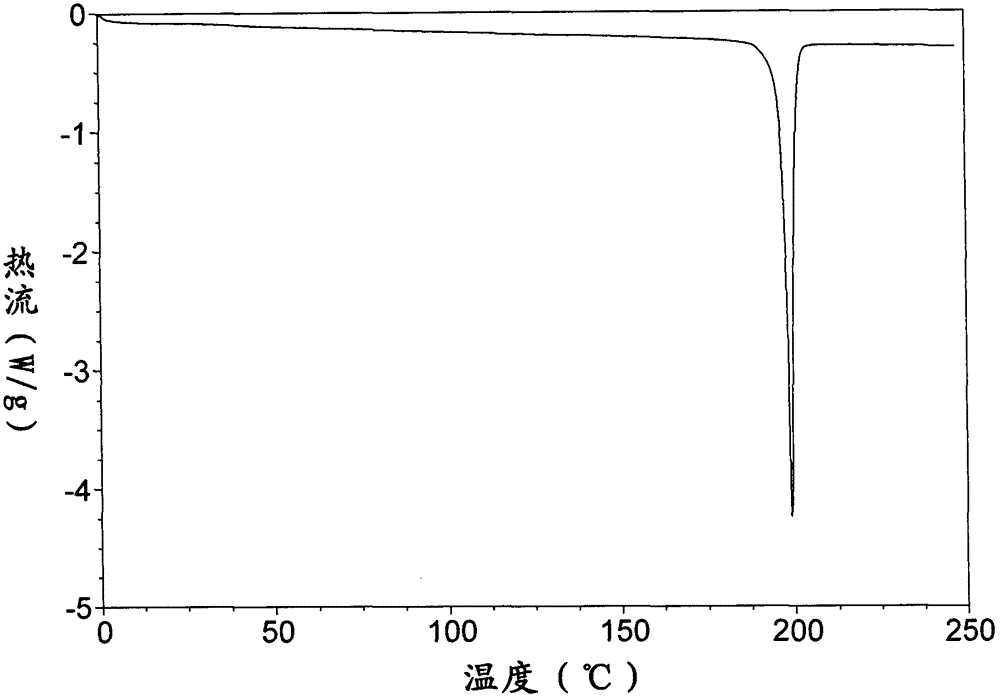
[0123] DSC spectrum such as Figure 7 As shown, it is amorphous, with a glass transition temperature of 46°C and a crystal transition at 137°C. After detection, crystal form I was formed after crystallization.
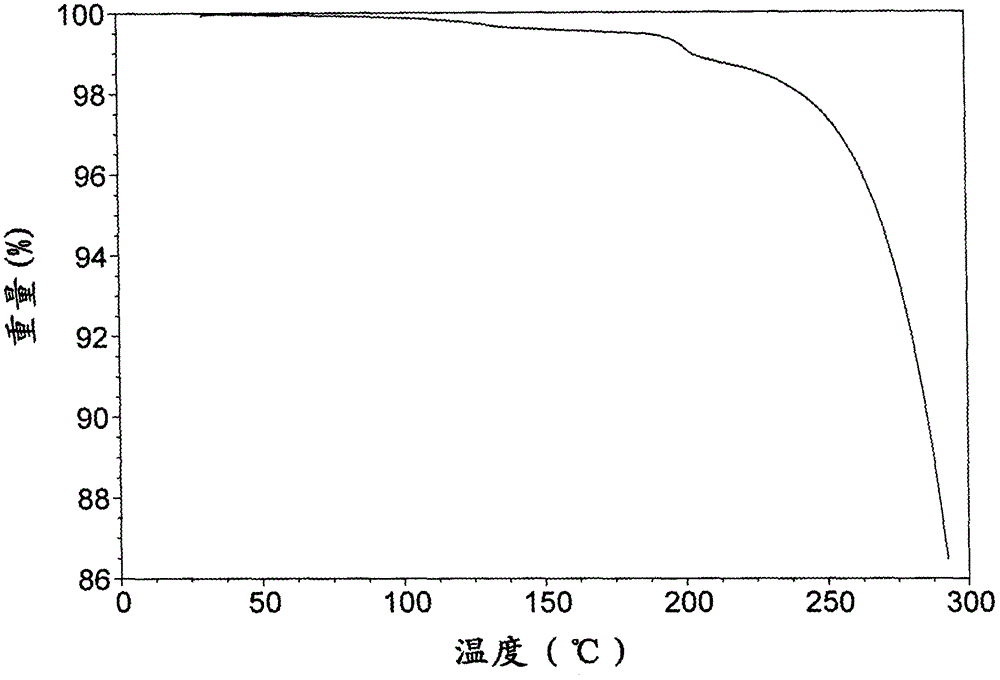
[0124] TGA spectrum such as Figure 8 shown.
[0125] IR spectrum such as Figure 9 shown.
[0126] Raman spectrum such as Figure 10 shown.
Embodiment 3
[0128]Take 10 mg of enzalutamide prepared in Example 1 and put it into a 30 mL single-necked flask, add 10 mL of dichloromethane, ultrasonicate at 40KHz for 20 seconds to ensure that it is completely dissolved, then place it on a rotary evaporator, spin dry at 40°C, and remove the solvent The rate is about 5 ml / min, resulting in amorphous enzalutamide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com