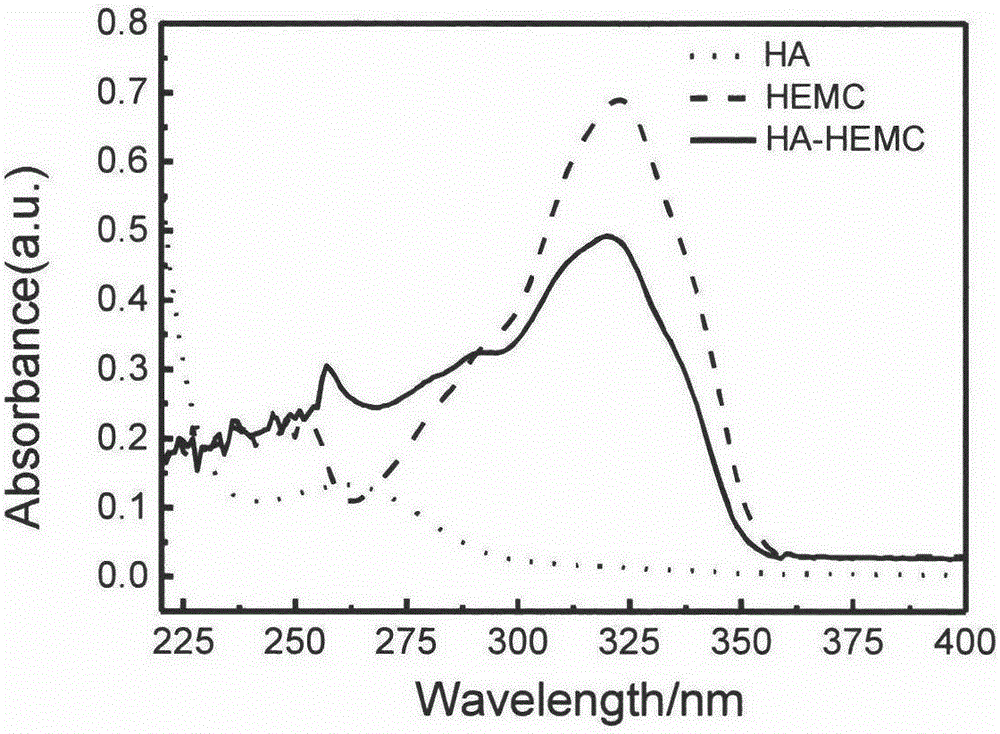
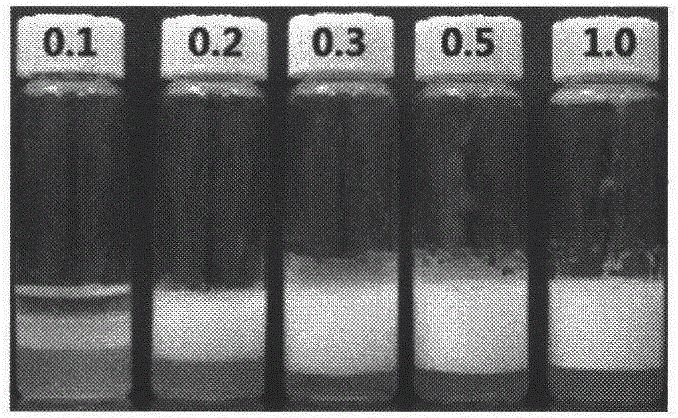
Particle emulsifier based on colloid self-assembled by hydrophobic modified hyaluronic acid and preparation method of particle emulsifier
A granular emulsifier and hyaluronic acid technology, applied in skin care preparations, cosmetics, etc., can solve problems such as vacancies, and achieve good emulsification effect, good stability, and high added value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of Hyaluronic Acid and Tetrabutylammonium Hydroxide Complex (HA-TBA)
[0029] 1.0g sodium hyaluronate (molecular weight is 11kDa) is dissolved in deionized water to prepare a 1wt% solution, stirred at room temperature until completely dissolved, and 3.0g strongly acidic cation exchange resin (H type, IR-120 ), after stirring for 5 hours, filter to remove the strongly acidic cation exchange resin; use 25% tetrabutylammonium hydroxide to adjust the pH of the filtrate to 7.0-7.03; freeze-dry to obtain the complex of hyaluronic acid and tetrabutylammonium hydroxide.
[0030] (2) Preparation of 7-(2-hydroxyethoxy)-4-methylcoumarin (HEMC)
[0031] Dissolve 5.0g of 7-hydroxy-4-methylcoumarin, 5.0g of 2-bromoethanol, and 3.0g of potassium carbonate in 50mL of ethanol, and reflux at 85°C for 24 hours; after the reaction, dissolve potassium carbonate in water and extract with ether; The organic layer was dried with anhydrous magnesium sulfate, and the solid was r...
Embodiment 2
[0042] (1) Preparation of hyaluronic acid and tetrabutylammonium hydroxide compound (HA-TBA), the method is the same as step (1) in Example 1.
[0043] (2) Preparation of 7-(2-hydroxyethoxy)-4-methylcoumarin (HEMC), the method is the same as step (2) in Example 1.
[0044] (3) Synthesis of HEMC modified hyaluronic acid
[0045]Weigh 0.5g of HA-TBA and dissolve it in 10mL of anhydrous DMSO. After complete dissolution, add 0.9898g of DCC and 0.2281g of DMAP, and stir at 60°C for 3h to activate the carboxyl group. Then 1.0736g HEMC was weighed and added into the reaction solution, and reacted at 60°C for 48h.
[0046] The reaction solution was suction filtered to remove insoluble matter, the filtrate was precipitated with acetone, and the centrifugation was repeated twice; the solid obtained by centrifugation was dissolved in 10-15mL DMSO, and the solution was dialyzed with sodium chloride solution (MWCO=3500Da) for one day, and then dialyzed with deionized water Three to four ...
Embodiment 3
[0049] (1) Preparation of hyaluronic acid and tetrabutylammonium hydroxide compound (HA-TBA), the method is the same as step (1) in Example 1.
[0050] (2) Preparation of 7-(2-hydroxyethoxy)-4-methylcoumarin (HEMC), the method is the same as step (2) in Example 1.
[0051] (3) Synthesis of HEMC modified hyaluronic acid
[0052] Weigh 0.5g HA-TBA and dissolve it in 10mL anhydrous DMSO, add 0.6599gDCC and 0.2144gDMAP after complete dissolution, stir at 60°C for 3h to activate the carboxyl group; then weigh 0.6599gHEMC and add it to the reaction solution, react at 60°C 48h.
[0053] Processing method is the same as step (3) in embodiment one.
[0054] (4) The preparation of HA-HEMC colloidal particles is the same as step (4) in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com