Pharmaceutical composition containing difluprednate and preparation method thereof
The technology of a kind of difluprednate and composition is applied in the field of pharmaceutical composition containing difluprednate and its preparation, which can solve the problems of poor cloth delivery, poor compliance, increased side effects, etc., and achieve uniform drug distribution, foreign matter The effect of reducing sensation and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
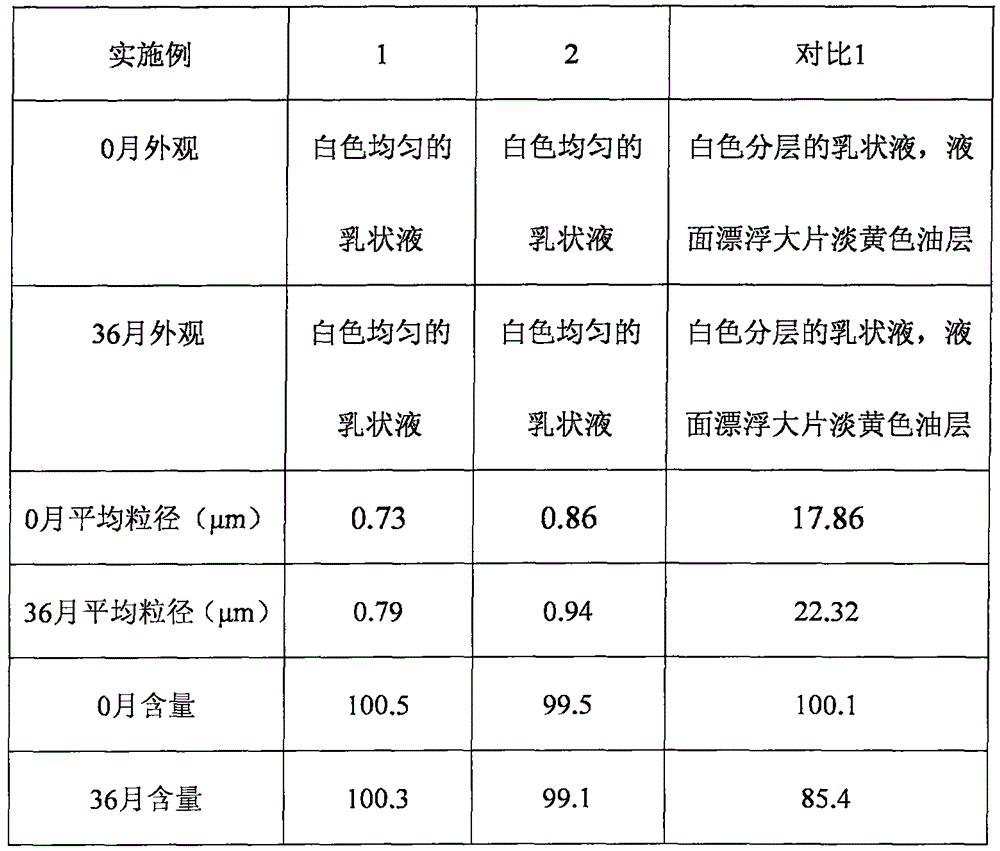
[0034] Purified water was heated to about 75°C, and glycerin, disodium edetate, sodium acetate, boric acid and sorbic acid were dissolved to obtain an aqueous phase. In addition, castor oil and poloxamer 407 were mixed and heated to about 75° C., and difluprednate was added to dissolve to obtain an oil phase. Then use a high-speed shearer to stir the water phase while slowly adding the oil phase to obtain colostrum, and adjust the pH to 5.5 after constant volume. The colostrum is processed by a high-pressure homogenizer, and then sterilized by moist heat to obtain an emulsion.
[0035] The particle size distribution table of difluprednate ophthalmic emulsion before the present embodiment is sterilized
[0036] particle size
Embodiment 2
100mL
[0040] Purified water was heated to about 75°C, and glycerin, disodium edetate, sodium acetate, boric acid and sorbic acid were dissolved to obtain an aqueous phase. In addition, soybean oil and egg yolk lecithin are mixed and heated to about 75° C., and difluprednate is added to dissolve to obtain an oil phase. Then use a high-speed shearer to stir the water phase while slowly adding the oil phase to obtain colostrum, and adjust the pH to 5.5 after constant volume. The colostrum is processed by a high-pressure homogenizer, and then sterilized by moist heat to obtain an emulsion.
[0041] The particle size distribution table of difluprednate ophthalmic emulsion after the present embodiment is sterilized
[0042] particle size
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com