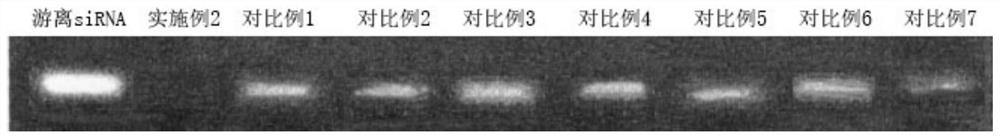
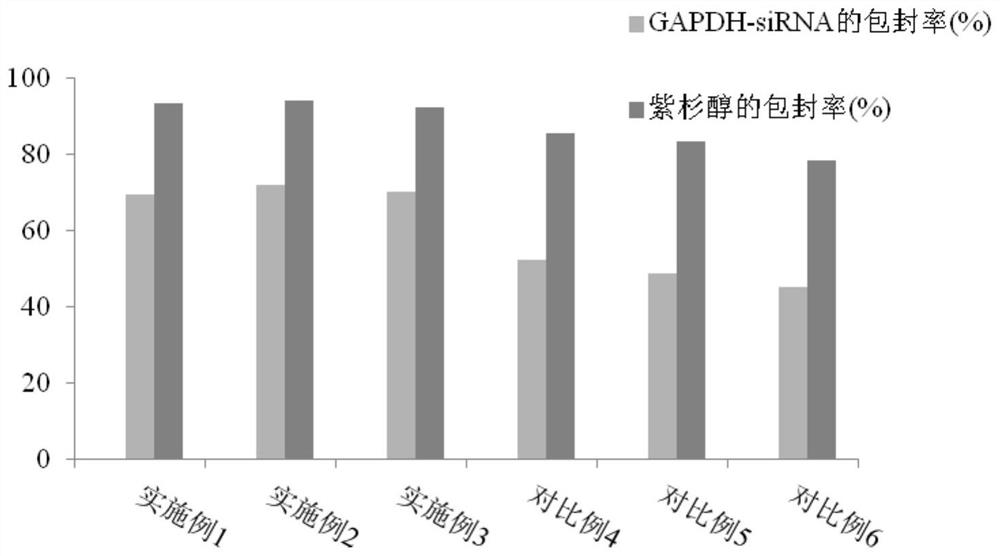
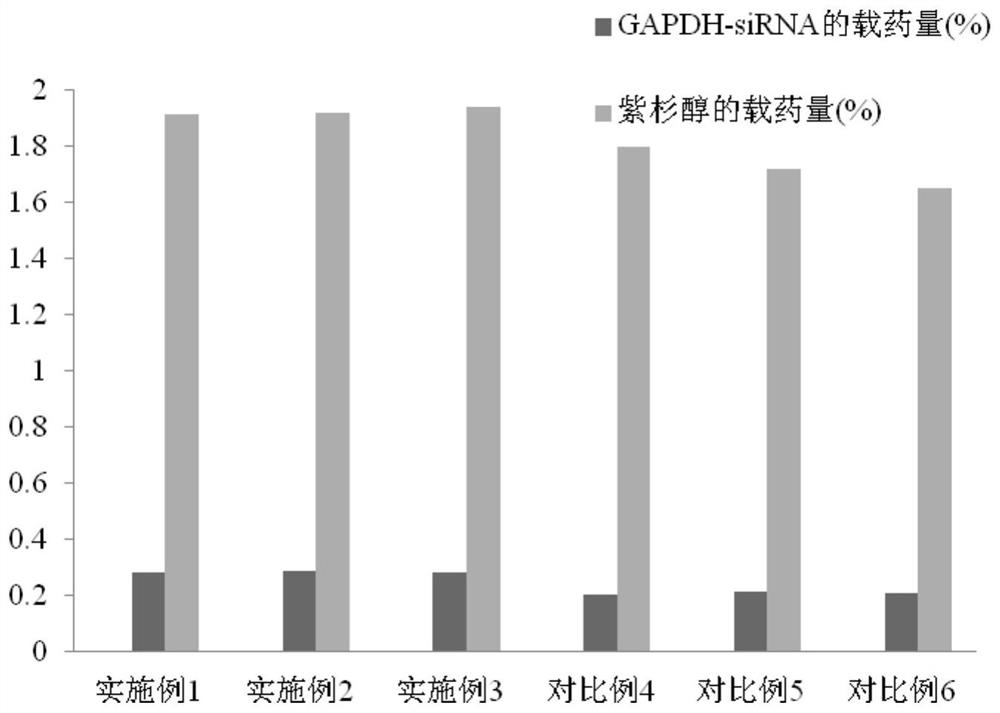
Preparation method of nanoparticle composition delivery system
A delivery system and nanoparticle technology, which can be used in drug combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of difficult to control the ratio of drug and gene load, and complex assembly process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A method of preparing a nanoparticle composition delivery system:
[0053] Cationic liposomes of antitumor drugs were prepared by thin film dispersion method: 2-dioleoyl hydroxypropyl-3-N,N,N-trimethylammonium chloride and phytosterols with a molar ratio of 1:0.8:0.2:2.8-3.2 , diacetone-D-galactose and dioleoyl phosphatidylethanol, paclitaxel with a mass ratio of 1:6 to dioleoyl phosphatidylethanolamine, alkyl glycosides with a hydrophilic-hydrophobic balance of 4.5 and guanidinoacetic acid , dissolved in absolute ethanol, put the solution into an eggplant-shaped bottle, and rotate it under reduced pressure in a water bath to form a uniformly distributed lipid film, remove the residual solvent, add RNase-free water, hydrate, and squeeze out.
[0054]GAPDH siRNA-paclitaxel liposomes were prepared by the mixed incubation method: Dissolve Cy3-labeled GAPDH-siRNA in RNase-free water, mix cationic liposomes and Cy3-labeled GAPDH-siRNA at a mass ratio of 18:1, and let stand a...
Embodiment 2
[0056] A method of preparing a nanoparticle composition delivery system:
[0057] Antitumor drug cationic liposomes were prepared by thin film dispersion method: 2-dioleoyl hydroxypropyl-3-N,N,N-trimethylammonium chloride, phytosterol, bis Acetone-D-galactose and dioleoyl phosphatidylethanol, paclitaxel with a mass ratio of dioleoyl phosphatidylethanolamine of 1:6.6, alkyl glycosides with a hydrophilic-hydrophobic balance of 4.8 and guanidinoacetic acid, dissolved In absolute ethanol, put the solution into an eggplant-shaped bottle, and rotate it under reduced pressure in a water bath to form a uniformly distributed lipid film, remove the residual solvent, add RNase-free water, hydrate, and squeeze out.
[0058] Prepare GAPDH siRNA-paclitaxel liposomes by mixed incubation method: Dissolve Cy3-labeled GAPDH-siRNA in RNase-free water, mix cationic liposomes and Cy3-labeled GAPDH-siRNA at a mass ratio of 19.3:1, and let stand at room temperature. Cy3-labeled GAPDH siRNA-paclitax...
Embodiment 3
[0060] A method of preparing a nanoparticle composition delivery system:
[0061] Cationic liposomes of antitumor drugs were prepared by film dispersion method: 2-dioleoyl hydroxypropyl-3-N,N,N-trimethylammonium chloride, phytosterols, bis Acetone-D-galactose and dioleoyl phosphatidylethanol, paclitaxel with a mass ratio of dioleoyl phosphatidylethanolamine of 1:7, alkyl glycosides with a hydrophilic-hydrophobic balance of 5.2 and guanidinoacetic acid, dissolved In absolute ethanol, put the solution into an eggplant-shaped bottle, and rotate it under reduced pressure in a water bath to form a uniformly distributed lipid film, remove the residual solvent, add RNase-free water, hydrate, and squeeze out.
[0062] GAPDH siRNA-paclitaxel liposomes were prepared by the mixed incubation method: Dissolve Cy3-labeled GAPDH-siRNA in RNase-free water, mix cationic liposomes and Cy3-labeled GAPDH-siRNA at a mass ratio of 22:1, and let stand at room temperature. Cy3-labeled GAPDH siRNA-pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com