Ent-kaurane diterpenoid compound and preparation method and application thereof
A kaurine type and kaurine technology, which is applied in the field of medicinal chemistry and can solve problems such as less phytochemical research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
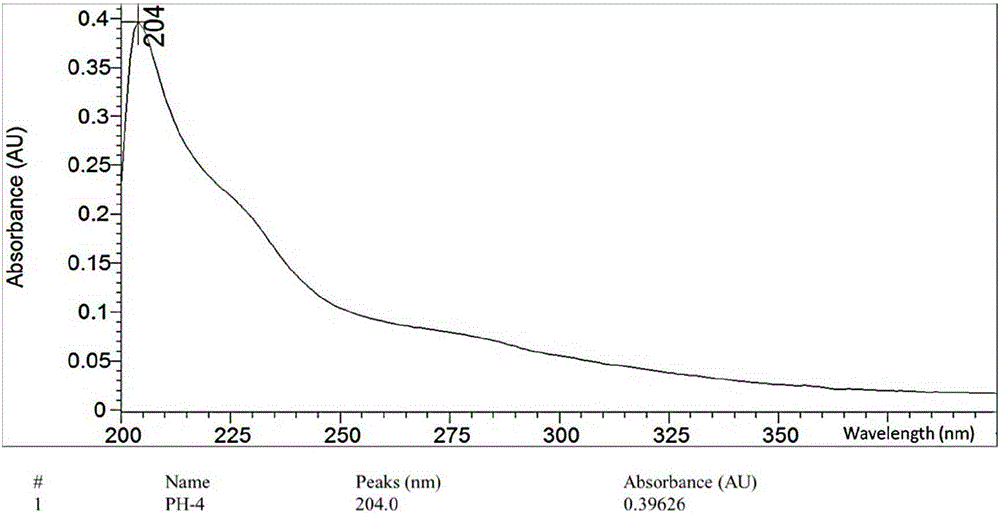
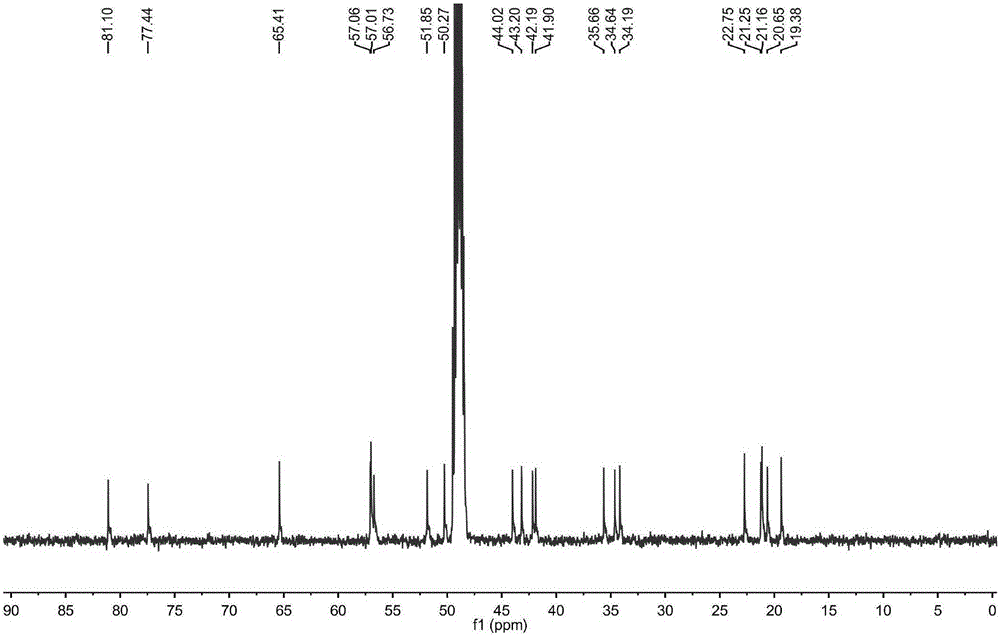
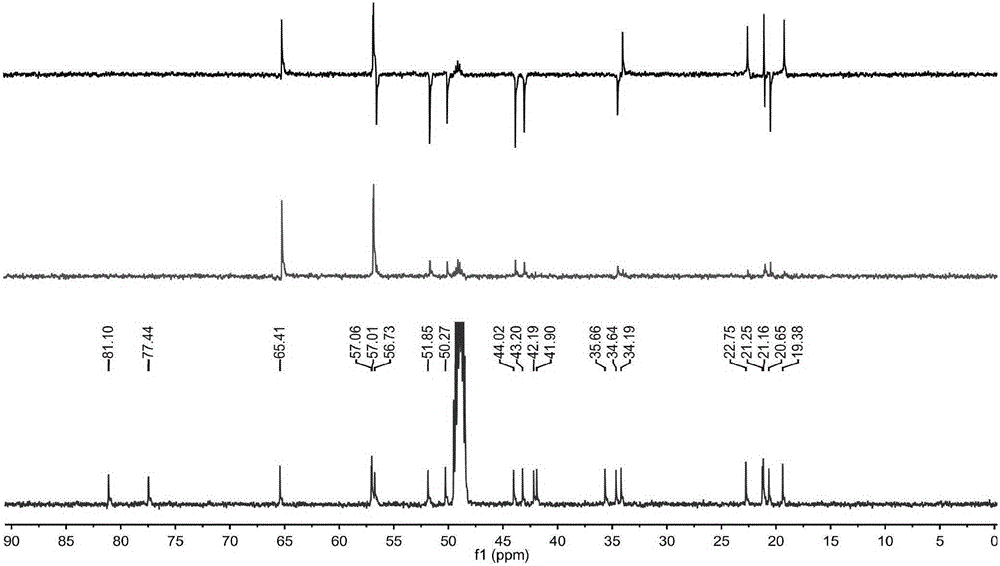
[0038] Determination of optical rotation Rudolph-type polarimeter (Rudolph Company, USA); hydrogen nuclear magnetic resonance spectrum 1 HNMR, carbon nuclear magnetic resonance spectrum 13 CNMR and 2DNMR were measured by JEOL500MHZ superconducting nuclear magnetic resonance spectrometer (tetramethylsilyl ether TMS as internal standard); high-resolution mass spectrum HR-SIMS was measured by VGAutospec-3000 mass spectrometer (at 70eV); ultraviolet spectrum UV was measured by HP8453 ultraviolet-visible spectrometer Photometer (Hewlett-Packard Company) is measured; Infrared spectrum IR is measured by VECTOR22 type Fourier transform infrared spectrometer (Germany BRUKER Instrument Company, KBr tablet); Melting point is measured by XT-4 binocular micro melting point measuring instrument (Beijing Tektronix Instrument Co., Ltd.) ; Silica gel for column chromatography (200-300 mesh) and silica gel for thin layer chromatography are produced by Qingdao Ocean Chemical; other reagents are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com