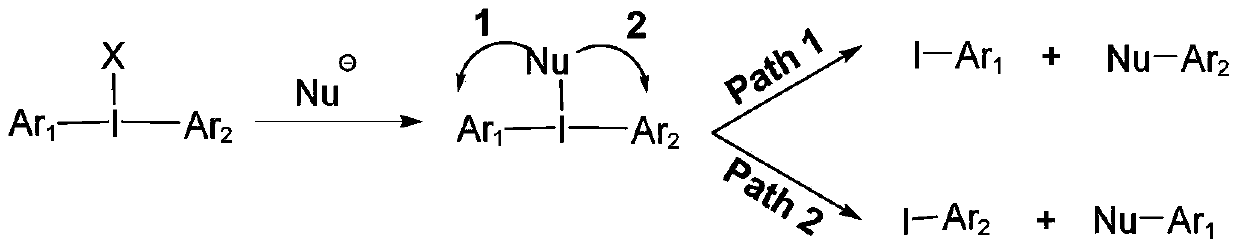
A kind of symmetrical pyrimidyl iodonium salt and preparation method thereof
A technology of pyrimidinyl and iodonium salts, which is applied in the field of iodonium salts and its preparation, can solve problems such as poor selectivity, low yield, and difficult separation, and achieve the effect of avoiding poor selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
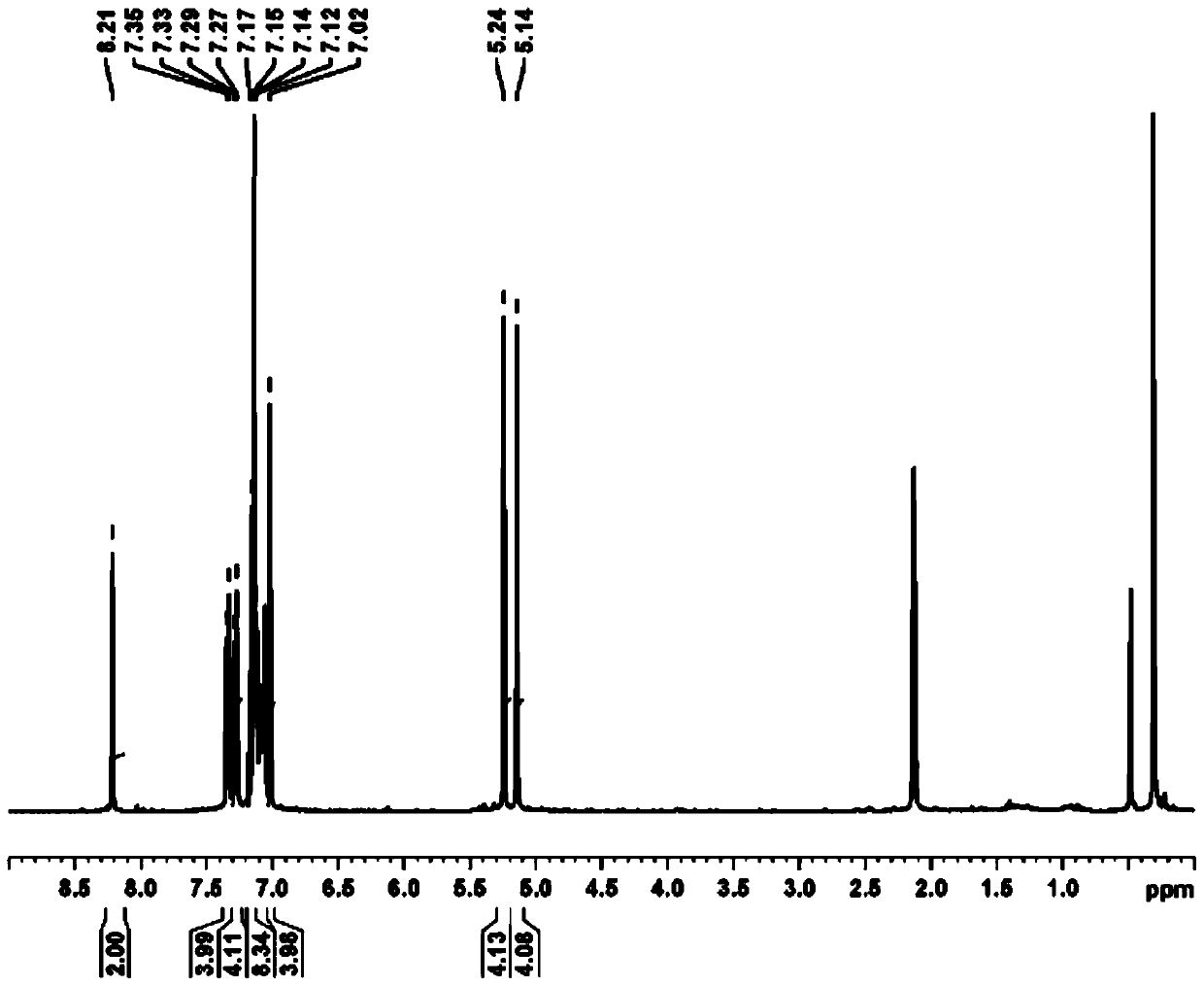
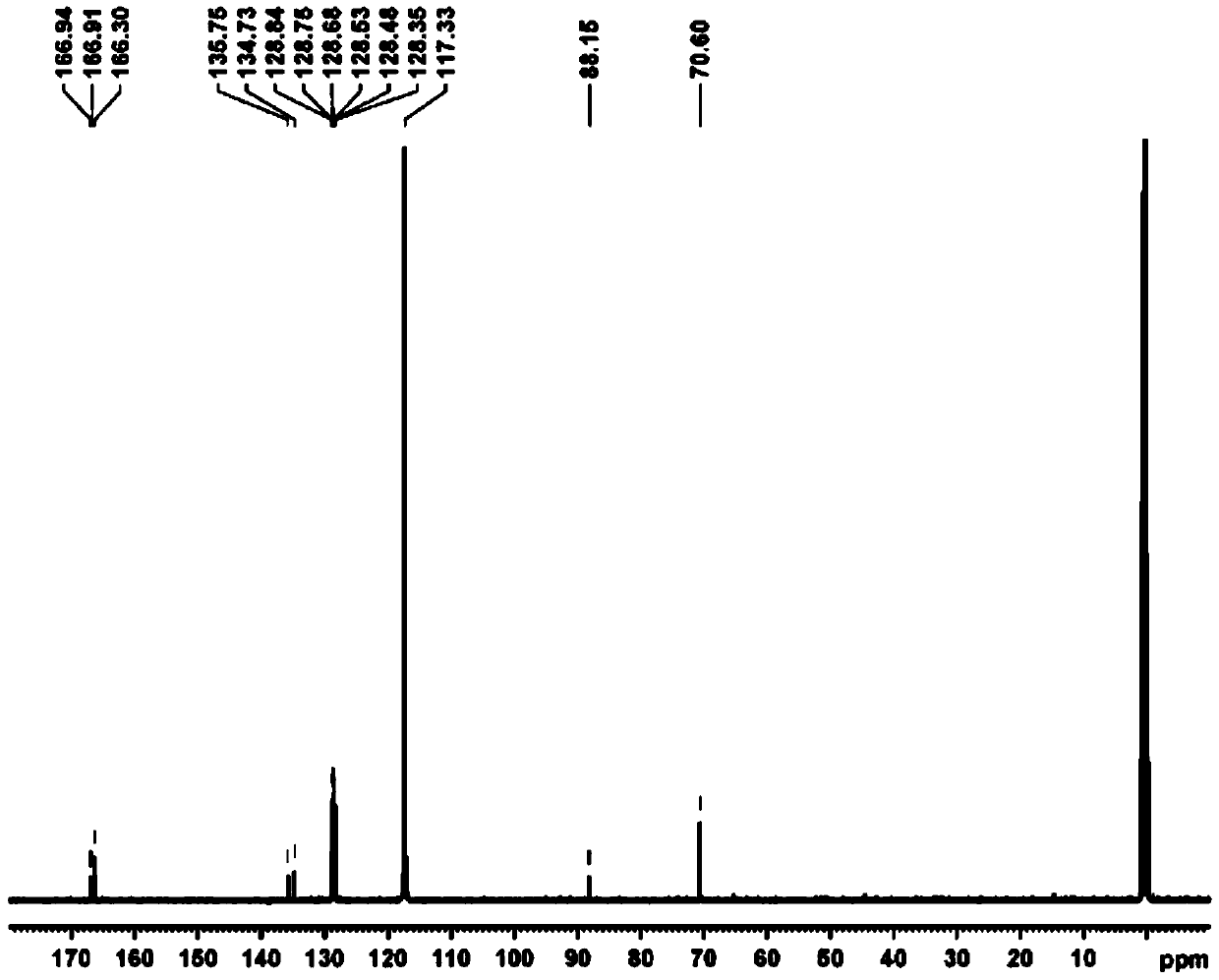
[0040] The preparation of bis(5-(2,4-bis(2,4-dibenzyloxy)pyrimidinyl)iodonium hexafluorophosphates), the structure is shown below :
[0041]
[0042] (1) Preparation of 5-bromo-2,4-dioxypyrimidine
[0043] 19.1 g (100 mmol) of 5-bromouracil was mixed with 60 ml of phosphorus oxychloride, and the reaction was stirred at 125° C. for five days, and 100 ml of ice water was added to quench the reaction. The reaction solution was extracted three times with 120 ml of diethyl ether, the organic phases were combined and distilled under reduced pressure to remove diethyl ether and excess phosphorus oxychloride to obtain 19.4 g (87%) of 5-bromo-2,4-dichloropyrimidine. Dissolve 6.7ml (65mmol) of benzyl alcohol in 70ml of anhydrous toluene, and react with 2.9g of NaH (60mmol) under the protection of nitrogen at 50°C until no gas is generated. The resulting suspension is cooled to room temperature and slowly added dropwise under stirring. 5-Bromo-2,4-dichloropyrimidine 4.5g (20mmol), the dripp...
Embodiment 2
[0051] Preparation of bis(5-(2,4-bis(2,4-dibenzyloxy)pyrimidinyl)iodonium tosylate) (bis(2,4-bis(benzyloxy)pyrimidin-5-yl)iodonium tosylate), the structural formula is as follows:
[0052]
[0053] (1) Preparation of 5-bromo-2,4-dioxypyrimidine: same as the first step (1) in Example 1.
[0054] (2) Preparation of 5-(2,4-dibenoxy-pyrimidinyl) potassium difluoroborate and 5-iodo-2,4-dibenoxypyrimidine
[0055] In a Schlenk flask, 2.91g (10mmol) of 2,4-dioxypyrimidine was dissolved in 50ml of anhydrous tetrahydrofuran, and 6.5ml of tert-butyllithium n-hexane solution (1.7 M, 1.12 equivalent) 4.5ml, keep -78℃ and continue to stir for 30 minutes, slowly add 2.26g (12mmol) of triisopropyl borate to the reaction solution dropwise, warm to room temperature, continue to stir and react for 8 hours, add 0.2mol / L hydrochloric acid was stirred for two hours. The reaction solution was extracted three times with 90ml of dichloromethane. The organic phases were combined and dried to remove the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com