Wolfberry lycopene epsilon-cyclase gene and recombinant vector comprising gene
A technology of lycopene and recombinant vector, which is applied in the fields of genetic engineering, plant gene improvement, recombinant DNA technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Lycium barbarum lycopene ε-cyclase gene Lm Clone of LcyE:
[0033] Using RNeasyPlantMiniKit (QIAGEN, German) kit, extract TotalRNA from 100mg fresh wolfberry leaves (Ningxia wolfberry), design the upstream primers according to the Unigene sequence of the transcriptome, Lm The upstream primers of LcyE gene are: Lm LcyEF: 5 ’ -ATGGAATGTGTTGGAGTTCAAAATT-3 ’ . The complete gene sequence was amplified using 3'-FULLRACECoreSetVer.2.0 (TaKaRa, Japan) kit. Specific steps: ① Use TotalRNA as a template and use 3'RACEAdaptor primers for reverse transcription reaction to synthesize 1stStrandcDNA. The reaction system is as follows:
[0034] RNA: 2μl
[0035] 3'RACEAdaptor: 1μl
[0036] 5×M-MLVBuffer: 2μl
[0037] dNTPMixture: 1μl
[0038] RNaseInhibitor: 0.25μl
[0039] ReverseTranscriptaseM-MLV: 0.25μl
[0040] RNaseFreeddH 2 O: 3.5μl
[0041] Reaction conditions: 42°C, 60min; 70°C, 15min.
[0042] ②According to the upstream primer of the gene and the downstream primer 3'RACEoutprimer prov...
Embodiment 2
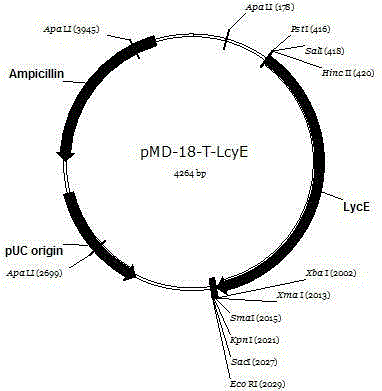
[0053] Cloning vector pMD-18- Lm The construction process of LcyE
[0054] As shown in the sequence table LmLm The LcyE gene is connected to the pMD-18 vector, and the reaction system is as follows:
[0055] Target PCR fragment: 4μl
[0056] pMD-18 vector: 1μl
[0057] SolutionI: 5μl
[0058] Reaction conditions: 16°C, 30 min. Conversion of ligation products E-Coli. TOP10, spread on the LB plate containing ampicillin. Perform PCR with the target gene as primer (reaction conditions: 94°C, 3min; 94°C, 30sec; 54°C, 30sec; 72°C, 2min30sec; 72°C, 10min, 30 cycles.) The PCR product was obtained, and the electrophoresis band was correct. Then it was sent to Huada Gene Sequencing Company for sequencing, and the sequencing result was Blasted in NCBI, indicating that it was the gene.
Embodiment 3
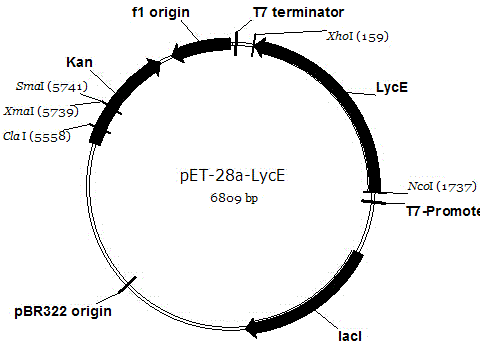
[0060] Escherichia coli expression vector pET28a -Lm The construction process of LcyE
[0061] First, take pMD-18- Lm LcyE is the template, using the upstream primer F2 shown in the sequence SEQIDNO.5 and the downstream primer R2 shown in the sequence SEQIDNO.6 as primers for amplification Lm The reaction system of LcyE fragment is:
[0062] ddH 2 O18.25μl
[0063] Taq-plusreactionBuffer2.5μl
[0064] dNTP2.0μl
[0065] PrimerF20.5μl
[0066] PrimerR20.5μl
[0067] Template1μl
[0068] Enzyme 0.25μl
[0069] The reaction conditions were 94°C, 4min; 94°C, 30sec; 55°C, 30sec; 72°C, 2min; 72°C, 10min, 30 cycles. The PCR product and pET28a plasmid were Noc I and Xho I double digestion, connect the two digestion products, the reaction system is:
[0070] 5×T4buffer2μl
[0071] pET28a (after digestion and purification) 5μl
[0072] Lm LcyE (after digestion and purification) 1μl
[0073] T4Enzyme 1μl
[0074] ddH 2 O1μl
[0075] The reaction conditions are 16°C, 16hrs.
[0076] The ligation produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com