Catalysts and methods for alcohol dehydration
A technology for dehydration catalysts and compounds, applied in chemical instruments and methods, catalyst regeneration/reactivation, physical/chemical process catalysts, etc., can solve problems such as expensive, difficult to handle, and rapid deactivation of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation of the dehydration catalyst can be performed such that it provides a BET surface area high enough to enable commercially viable product yields. Synthetic methods known to those skilled in the art can be performed to maximize the active surface area for selective production of the desired product. These methods include, but are not limited to, sol-gel preparation, flame pyrolysis, colloidal routes, molding methods, and grinding. Additionally, compounds such as, but not limited to, sacrificial porogens, structure directing compounds, stripping agents, and / or pillaring agents may be added to increase surface area. The BET surface area of the dehydration catalyst is preferably greater than 5m 2 / g, more preferably greater than 50m 2 / g, and more preferably greater than 150m 2 / g.
[0026] The dehydration catalyst in the reaction vessel may optionally contain a binder and / or matrix material other than the oxides of rare earth elements. Non-limiting exa...
example 1
[0058] Synthesis of Lanthanum Oxychloride by Thermal Decomposition of LaCl 3 ·7H 2 O execute. A sample (about 10 g) of the powdered precursor was calcined in air in a static calcination furnace with the following temperature profile: 1.41 °C / min ramp to 550 °C, 3 hour dwell at 550 °C, cooling to room temperature. The elemental composition of the catalyst was analyzed by X-ray fluorescence spectroscopy (XRF) to be 17.23 wt.% chlorine, 69.63 wt.% lanthanum and 13.14 wt.% oxygen (remainder). Therefore, the elemental composition of the catalyst is La 1.00 o 1.64 Cl 0.97 . The specific surface area (BET) of the catalyst sample was measured to be 6.2m 2 / g and its pore volume is 0.013cm 3 / g. XRD data showed the presence of a lanthanum oxychloride phase.
example 2
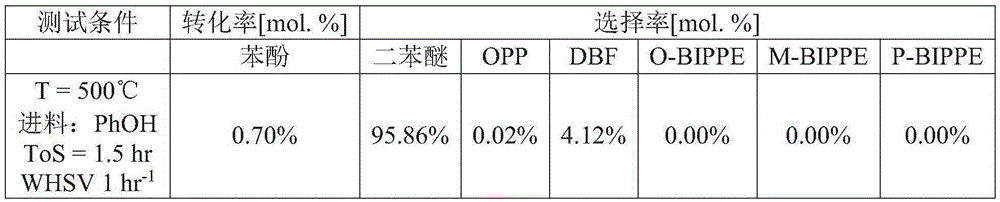
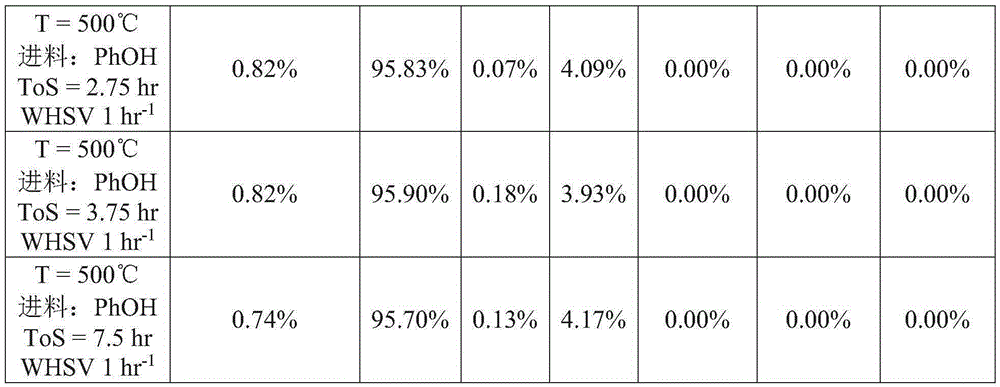
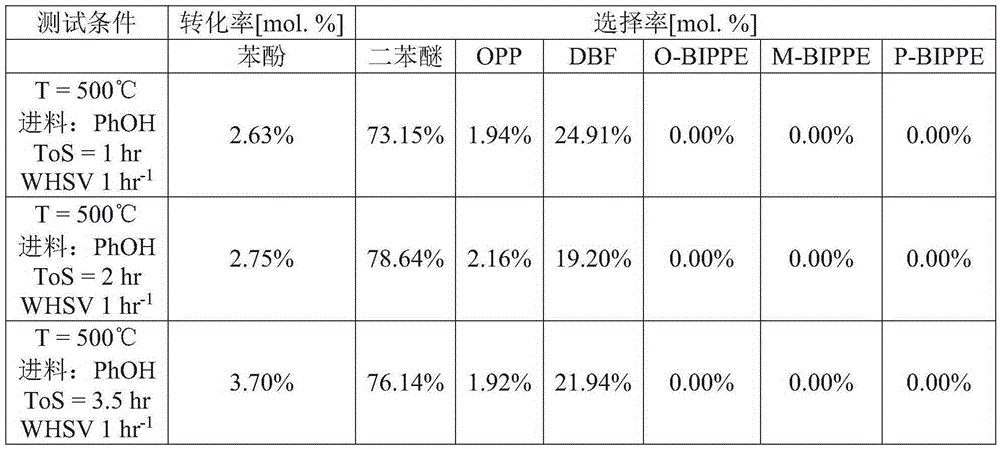
[0060] The lanthanum oxychloride catalyst from Example 1 was used for the dehydration of phenol. The powder is pressed and sieved to obtain particles with a diameter of 0.60 mm to 0.85 mm. Particles were loaded into an electrically heated stainless steel reactor tube and heated to reaction temperature with nitrogen gas flowing through the tube. After reaching the reaction temperature, gas phase phenol was passed through the reactor tube. The conversion of phenol was performed at 1 weight hourly space velocity (WHSV=g phenol / g catalyst-hour) and at 500°C. Test conditions and results are shown in Table 1.
[0061] Table 1
[0062]
[0063]
[0064] OPP: o-phenylphenol. DBF: Dibenzofuran. O-BIPPE: o-biphenylphenyl ether. M-BIPPE: m-biphenylphenyl ether. P-BIPPE: p-biphenylphenyl ether. PhOH: phenol. N2: Nitrogen. ToS: Reaction time (defined as ToS = 0 hours at the start of phenol flow).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com