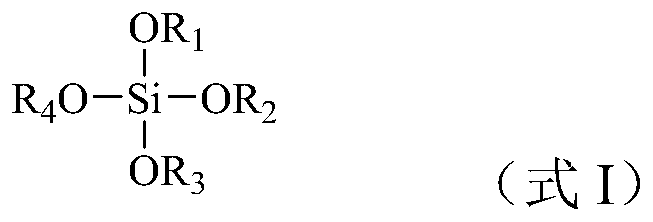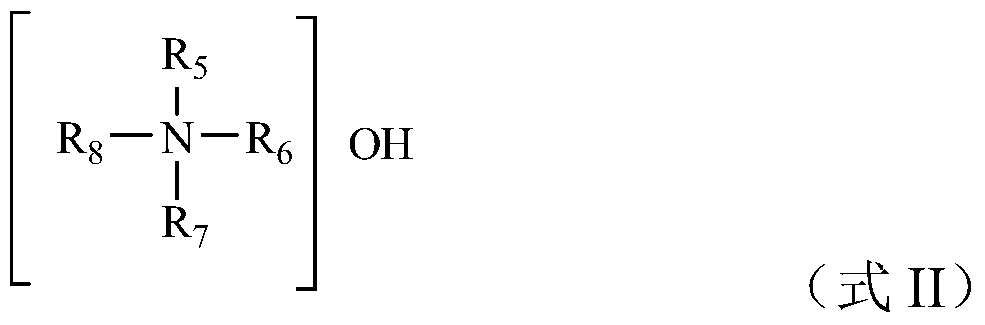Tin-silicon molecular sieve and synthetic method and application thereof, and phenol hydroxylation method
A tin-silicon molecular sieve and phenol hydroxylation technology are applied in the direction of molecular sieve catalysts, chemical instruments and methods, and the preparation of organic compounds, which can solve problems such as low yield, long crystallization time of tin-silicon molecular sieves, and weak crystal guiding ability. Achieve the effects of high crystallinity, shorten hydrolysis time, and reduce dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Firstly, tetraethyl orthosilicate as a source of organosilicic acid and tin chloride pentahydrate as a source of tin are dissolved in an aqueous solution of tetrapropylammonium hydroxide as an alkali source template agent, wherein the molar composition of the material is organosilicon source: tin source: alkali source Templating agent: water = 100:5:10:400, organic silicon source is SiO 2 Meter, tin source as SnO 2 Meter, alkali source template with OH - After the silicon ester is hydrolyzed (the hydrolysis rate is 50%), the mixture is transferred to a sealed reactor for hydrothermal crystallization at 140°C for 6 hours. After cooling, the reactor is opened to add silica gel A to the crystallization system and mix well. SiO 2 According to the calculation, the molar ratio of the added silica gel to the organic silicon source was 1:0.2, and then the mixture was continued to crystallize in a sealed reaction vessel at a temperature of 170°C and autogenous pressure for 12 ...
Embodiment 2
[0070] Firstly, the organosilicon source tetraethyl orthosilicate and the tin source tin chloride pentahydrate are dissolved in the alkali source template agent tetrapropyl ammonium hydroxide aqueous solution, wherein the material molar composition is organosilicon source: tin source: alkali source template agent : water = 100:2:15:500, organic silicon source is SiO 2 Meter, tin source as SnO 2 Meter, alkali source template with OH - After the silicon ester (hydrolysis rate is 70%) is hydrolyzed, the mixture is transferred to a sealed reactor for hydrothermal crystallization at 140°C for 10 hours. After cooling, the reactor is opened to add silica gel B to the crystallization system and mix well. SiO 2 Calculated, the molar ratio of the added silica gel to the organic silicon source is 1:0.1, and then the mixture is continued to be crystallized in a sealed reactor at a temperature of 170°C and an autogenous pressure for 20h, and then the crystallized product is recovered acc...
Embodiment 3
[0072] Firstly, the organosilicon source tetraethyl orthosilicate and the tin source tetrabutyl stannate are dissolved in the alkali source template agent tetrapropyl ammonium hydroxide aqueous solution, wherein, the molar composition of the material is organosilicon source: tin source: alkali source template Agent: water = 100:1:20:650, organic silicon source is SiO 2 Meter, tin source as SnO 2 Meter, alkali source template with OH - After the silicon ester is hydrolyzed (the hydrolysis rate is 60%), the mixture is transferred to a sealed reactor for hydrothermal crystallization at 120°C for 8 hours, and after cooling, the reactor is opened to add silica sol with a mass fraction of 25% to the crystallization system. and mix well, wherein the molar ratio of added silica sol to organosilicon source is 1:0.5 in terms of silicon dioxide, and then continue to crystallize the mixture in a sealed reaction kettle at a temperature of 160°C and autogenous pressure for 14h, and then T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com