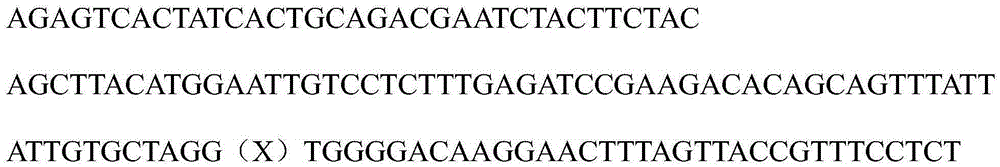Method for synthesizing controllable humanized antibody library based on combinatorial synthesis technique and application
A humanized antibody and combinatorial synthesis technology, applied in the field of genetic engineering, can solve the problems of narrow screening range of monoclonal antibody, low coupling efficiency of chemical synthesis, and affecting the preparation of high-affinity human monoclonal antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] This example discloses a method for preparing a humanized antibody library with controllable amino acid changes and quantity based on combinatorial synthesis technology. This example takes the sequence of CDR3 of the heavy chain variable region (VH1) of a human antibody as an example .
[0051] 1. Design humanized antibody library (VH1)
[0052] Human germline antibody gene VH1 family, its gene sequence is known and the utilization rate of this gene family in the immune response is high. In this example, the sequence synthesis of human antibody heavy chain variable region (VH1) CDR3 is taken as an example , the construction of the CDR3 library requires the convenient introduction of amino acids at any position or at any ratio; the amino acid sequence of the sequence to be synthesized in this example is represented by a single-letter amino acid:
[0053] QVQLVQSGAEVKKPGSSVKVSCKASGGTFSSYAISWVRQAPGQGLEWMGGIIPIFGTANYAQKFQGRVTITADESTSTAYMELSSLRSEDTAVYYCAR(X)WGQGTLVTVSS, in ...
Embodiment 2
[0099] Example 2 uses the method disclosed in Example 1 to synthesize specific required amino acids in a mammalian expression system at a required ratio.
[0100] 1. Design humanized antibody library (VH1)
[0101] Human germline antibody gene VH1 family, its gene sequence is known and the utilization rate of this gene family in the immune response is high. In this example, the sequence synthesis of human antibody heavy chain variable region (VH1) CDR3 is taken as an example , the construction of the CDR3 library requires the convenient introduction of amino acids at any position or at any ratio; the amino acid sequence of the sequence to be synthesized in this example is represented by a single-letter amino acid:
[0102] QVQLVQSGAEVKKPGSSVKVSCKASGGTFSSYAISWVRQAPGQGLEWMGGIIPIFGTANYAQKFQGRVTITADESTSTAYMELSSLRSEDTAVYYCAR(X)WGQGTLVTVSS, in order to reduce the number of groups, where X represents 14 random amino acids, and according to the combined synthesis method, each amino ac...
Embodiment 3
[0123] Example 3 Using the method disclosed in Example 1, amino acids were synthesized in a specific ratio in a yeast expression system.
[0124] 1. Design of humanized antibody library (VH1)
[0125] Human germline antibody gene VH1 family, its gene sequence is known and the utilization rate of this gene family is high in the immune response, the sequence of CDR3 of the heavy chain variable region of human antibody (VH1) is synthesized, and the amino acid sequence of the sequence to be synthesized is used Single-letter amino acids represent:
[0126] QVQLVQSGAEVKKPGSSVKVSCKASGGTFSSYAISWVRQAPGQGLEWMGGIIPIFGTANYAQKFQGRVTITADESTSTAYMELSSLRSEDTAVYYCAR(X)WGQGTLVTVSS, in order to reduce the number of groups, where X represents 14 random amino acids, requiring tyrosine at positions 13-14 of X, and arginine at a ratio of 80:20. See the implementation for the amino acid codon sequence Table 1 in Example 1.
[0127] 2. Combinatorial synthesis of humanized recombinant antibody library...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com