Composition for heat melt extrusion and method for producing heat melt extruded product using same
A hot-melt extrusion and composition technology, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problem of drug inactivation, easy hydrolysis and deactivation of drugs or carriers problem to achieve high bioabsorbability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 5 and comparative example 1 to 2
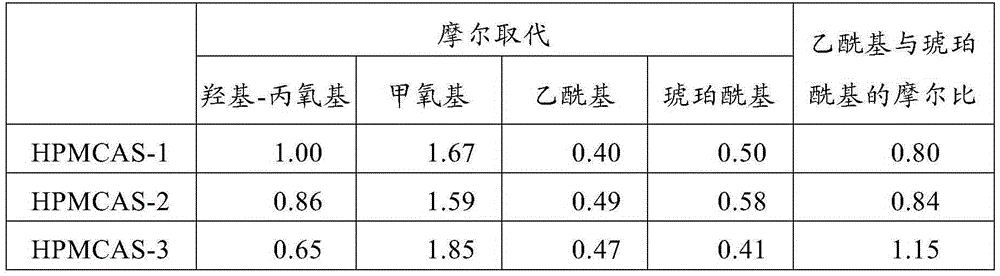
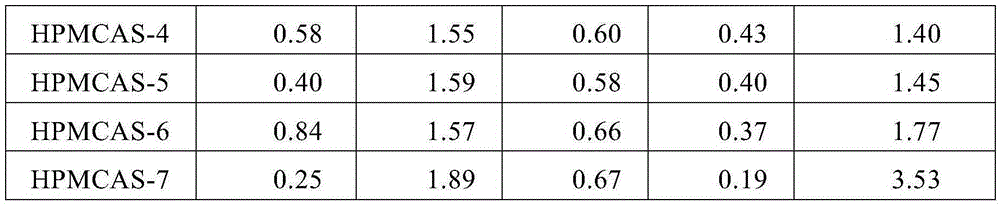
[0095] HPMCAS-1 to HPMCAS-7 were pre-dried such that the moisture content was measured to be less than 1 wt%. Using a die diameter of 1 mm, a die height of 10 mm, and an extrusion rate of 50 mm / min, extruded from a die outlet in a vacuum extruder (single-screw piston type melt extruder: Capilograph manufactured by Toyo Seiki Seisaku-sho, Ltd.) The dried HPMCAS-1 to HPMCAS-7 were taken out, and the minimum extrusion temperature of HPMCAS-1 to HPMCAS-7 was measured. The results are shown in Table 3.
[0096] table 3
[0097]
HPMCAS
Glass transition temperature (°C)
Minimum extrusion temperature (°C)
Example 1
HPMCAS-1
70
110
Example 2
HPMCAS-2
81
140
Example 3
HPMCAS-3
94
140
Example 4
HPMCAS-4
101
140
Example 5
HPMCAS-5
112
150
Comparative example 1
HPMCAS-6
85
140
Comparative example 2
HPMCAS-7
126
180
[0098] Th...
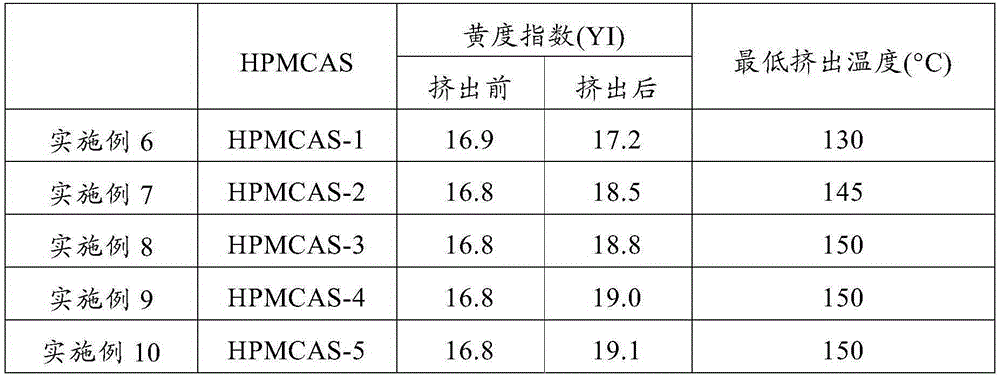
Embodiment 6 to 10 and comparative example 3 to 4
[0101] Hot melt extrudates were prepared using the poorly water soluble drug ascorbic acid. Ascorbic acid has a thermal decomposition temperature of 176°C and is a model drug, which raises concerns about inactivation due to thermal decomposition during hot-melt extrusion.
[0102] The respective HPMCAS and ascorbic acid powders were mixed in a mortar at a weight ratio of HPMCAS to ascorbic acid of 1:0.5 to prepare compositions for hot melt extrusion.
[0103] Subsequently, a hot-melt extruder (Thermo Fisher Scientific Inc. The HAAKEMiniLab produced) makes the above mixed powder carry out hot-melt extrusion. The minimum extrusion temperature of the hot-melt extrudate was measured in the same manner as in Example 1. Further, the hot-melt extrudate thus obtained was pulverized at 20000 rpm with a pulverizer (WonderBlender WB-1 produced by Osaka Chemical Co., Ltd.) and sieved through a 30-mesh sieve (opening of 500 μm). The powder thus obtained and the composition for hot-melt ...
Embodiment 11 to 15 and comparative example 5 to 6
[0109] Film test pieces were prepared by using HPMCAS-1 to HPMCAS-7 and subjected to measurement of dissolution time in phosphate buffer. A solution of 16 g of HPMCAS in 64 g of a mixed solvent of dichloromethane and ethanol in a 1:1 weight ratio of dichloromethane to ethanol was cast on a glass plate and dried at room temperature. The resulting film was dried at 80° C. for 2 hours, and cut into test pieces having a thickness of 100 μm, a length of 1 cm, and a width of 1 cm.
[0110] The dissolution time of the test piece was measured according to the disintegration test (auxiliary tube) in the 16th edition of the Japanese Pharmacopoeia. More specifically, one of the test pieces was placed in 1 L of phosphate buffer solution with a pH of 6.0, and the test piece was dissolved to A measurement of the time required to observe the absence of undissolved material. The phosphate buffer has a pH of 6.0, which corresponds to the pH of the digestive fluid in the upper to middle part ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com