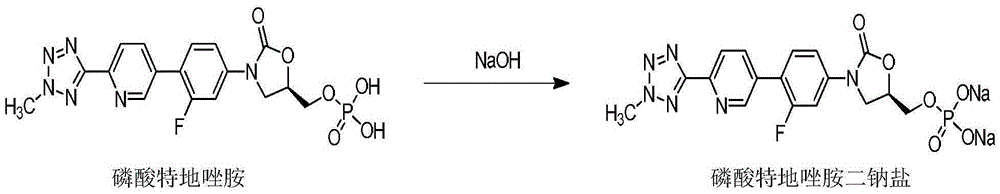
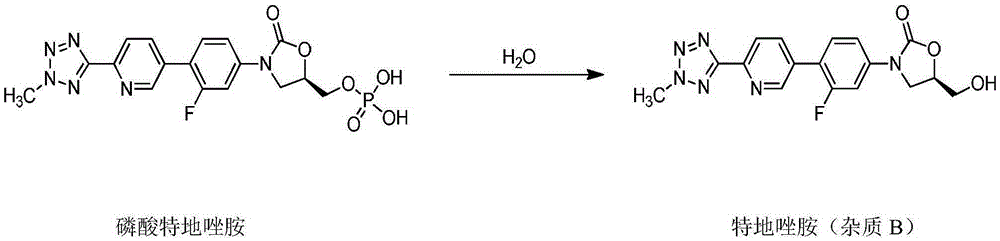
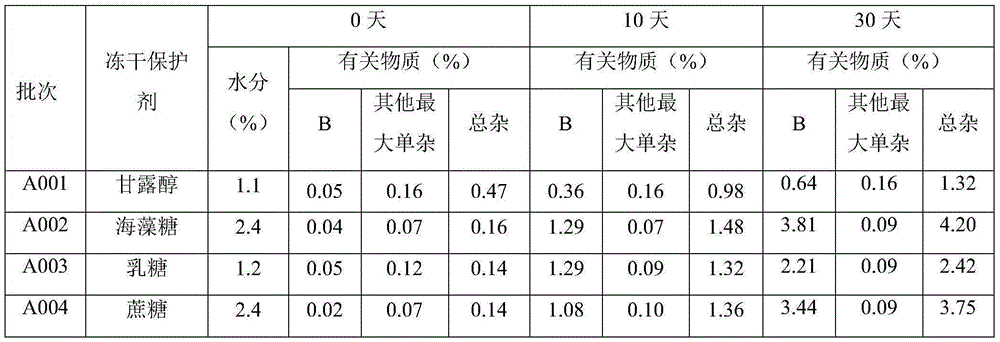
Tedizolid phosphate for injection
A technology for tedizolid phosphate and injection, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, freeze-dried delivery, etc. It can solve the problems of high moisture content, unqualified product quality, and drug stability. Not advanced question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The tedizolid phosphate for injection of the present invention has a prescription composition as follows:
[0034] Composition
Dosage
effect
210mg
Active ingredients
70mg
Protective agent
35mg
Protective agent
Sodium Hydroxide and Hydrochloric Acid
Adjust pH to 7.0-8.0
pH regulator
Water for Injection
2.1ml
[0035] Its preparation method comprises the following steps:
[0036] Add 60% of the prescribed amount of water for injection to the liquid preparation tank, and cool to 30°C; add the prescribed amount of tedizolid phosphate, and add sodium hydroxide solution dropwise under stirring until the liquid medicine is clear. Add the prescribed amount of lactose and trehalose, stir to dissolve, adjust the pH value to 7.0-8.0 with sodium hydroxide solution or hydrochloric acid solution; add water for injection to make up to th...
Embodiment 2
[0039] The tedizolid phosphate for injection of the present invention has a prescription composition as follows:
[0040] Composition
Dosage
effect
210mg
Active ingredients
70mg
Protective agent
35mg
Protective agent
Sodium Hydroxide and Hydrochloric Acid
Adjust pH to 7.0-8.0
pH regulator
Water for Injection
2.1ml
[0041] Its preparation method comprises the following steps:
[0042]Add 60% of the prescribed amount of water for injection to the liquid preparation tank, and adjust the temperature to 40°C; add the prescribed amount of tedizolid phosphate, and add sodium hydroxide solution dropwise under stirring until the liquid medicine is clear. Add the prescribed amount of lactose and trehalose, stir to dissolve, adjust the pH value to 7.0-8.0 with sodium hydroxide solution or hydrochloric acid solution; add water for injection...
Embodiment 3
[0045] The tedizolid phosphate for injection of the present invention has a prescription composition as follows:
[0046] Composition
Dosage
effect
200mg
Active ingredients
lactose
50mg
Protective agent
50mg
Protective agent
Sodium Hydroxide and Hydrochloric Acid
Adjust pH to 7.0-8.0
pH regulator
Water for Injection
2ml
[0048] The specific preparation method comprises the following steps: adding 70% of the prescribed amount of water for injection to the liquid preparation tank, cooling to 20° C.; adding the prescribed amount of tedizolid phosphate, and adding sodium hydroxide solution dropwise under the condition of stirring until the medicinal liquid is clear. Add the prescribed amount of lactose and trehalose, stir to dissolve, adjust the pH value to 7.0-8.0 with sodium hydroxide solution or hydrochloric acid soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com