Preparation method and application of superparamagnetic Fe3O4 nano material
A nanomaterial and superparamagnetic technology, applied in the field of nanomaterials, can solve the problems of low purity, poor crystallinity and magnetic properties, and complicated preparation process of the hydrothermal reaction method, and achieve easy control of product composition, high adsorption performance, and conditions easy to control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0033] Preparation Example 1: Preparation of superparamagnetic Fe 3 O 4 nanomaterials
[0034] (1) According to the molar ratio of 0.5:5, the solid FeCl 3 ·6H 2 O was dissolved in distilled water, and magnetically stirred at room temperature for 2 minutes to fully dissolve it to obtain solution a.
[0035] (2) Slowly add the triethanolamine (TEA) solution to solution a, the volume ratio of triethanolamine (TEA) to distilled water is 10:1, continue magnetic stirring for 2 minutes to obtain brown-red solution b.
[0036] (3) Adjust the pH of the solution b to 10 with solid NaOH to obtain a dark brown solution c.
[0037] (4) Put solution c into the reaction kettle, react at 180°C for 1.5 hours, take out the product by magnetic separation, and dry the obtained product in an oven at 60°C for 4 hours to obtain superparamagnetic Fe 3 O 4 nanomaterials.
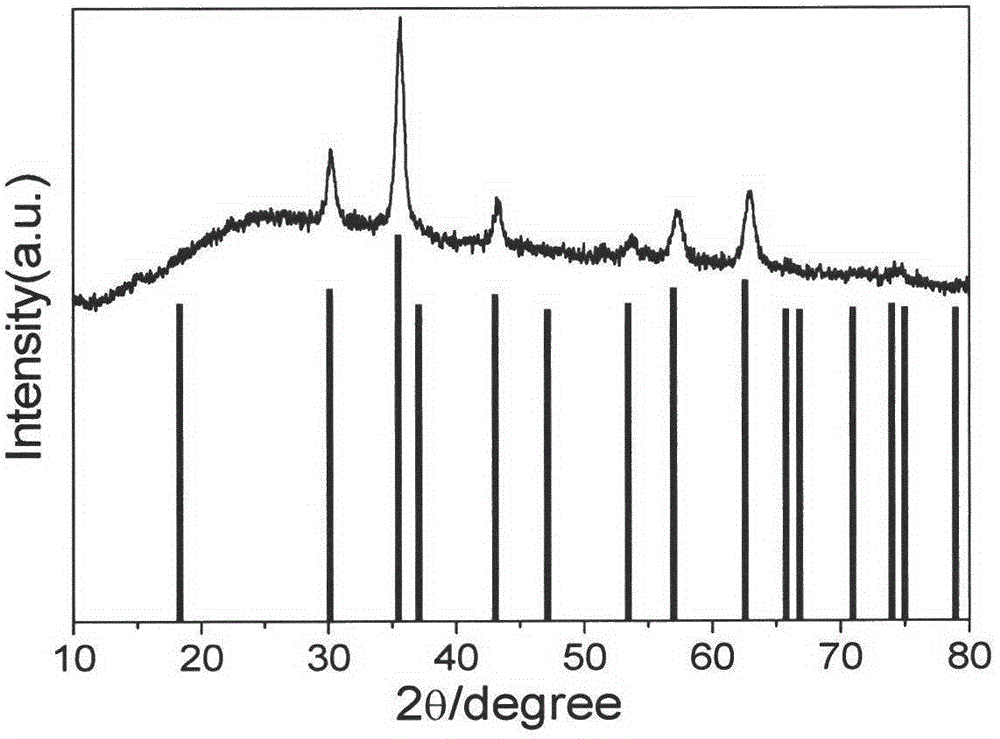
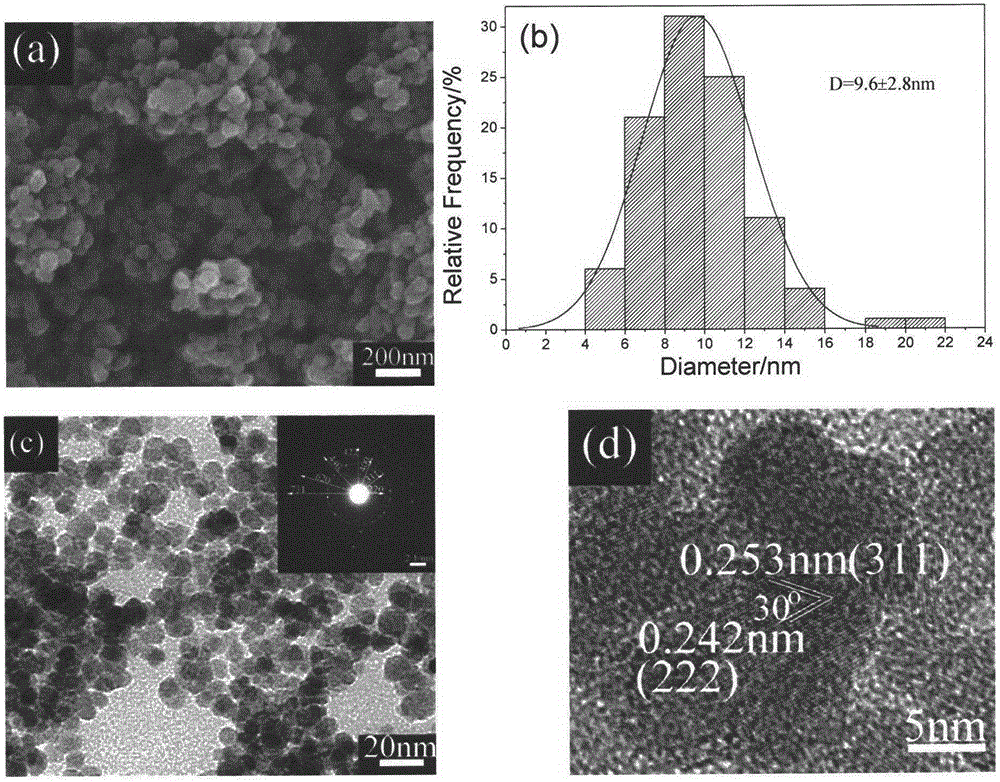
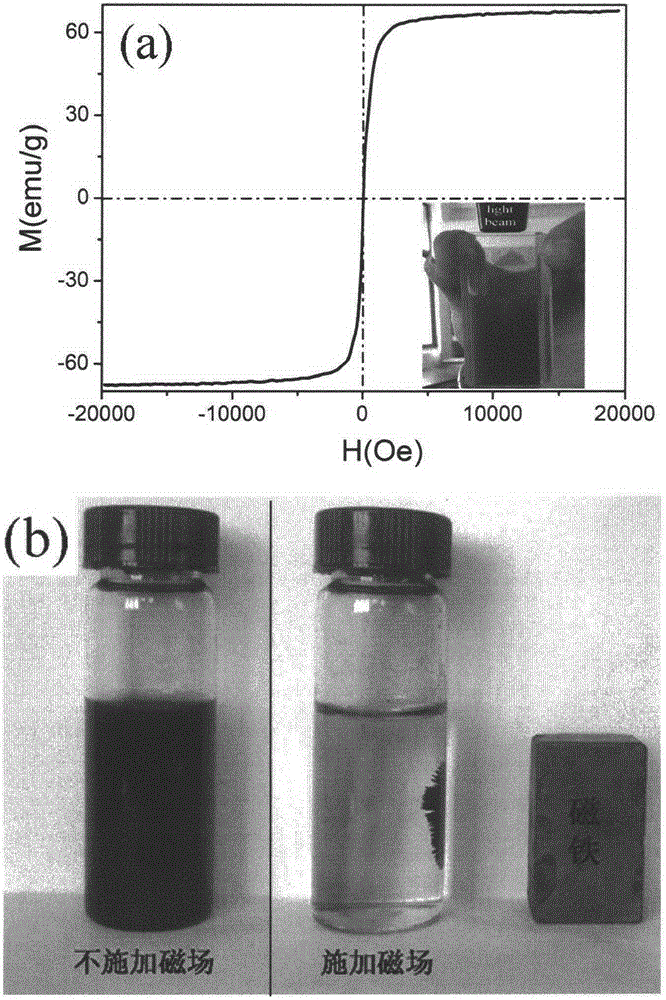
[0038] figure 1 Is the XRD spectrum of the product obtained in Example 1, and the standard Fe 3 O 4 The spectrum (JCPDS: 76-1849) is availab...
preparation Embodiment 2
[0043] Preparation Example 2: Preparation of superparamagnetic Fe 3 O 4 nanomaterials
[0044] (1) According to the molar ratio of 0.1:5, the solid FeCl 3 ·6H 2 O was dissolved in distilled water, and magnetically stirred at room temperature for 1 minute to fully dissolve it to obtain solution a.
[0045] (2) Slowly add the triethanolamine (TEA) solution to solution a, the volume ratio of triethanolamine (TEA) to distilled water is 10:1, continue magnetic stirring for 3 minutes to obtain brown-red solution b.
[0046] (3) Adjust the pH of the solution b to 9 with ammonia water to obtain a dark brown solution c.
[0047] (4) Put solution c into the reaction kettle, react at 200°C for 2 hours, take out the product by magnetic separation method, and dry the obtained product in an oven at 50°C for 6 hours to obtain superparamagnetic Fe 3 O 4 nanomaterials.
preparation Embodiment 3
[0048] Preparation Example 3: Preparation of superparamagnetic Fe 3 O 4 nanomaterials
[0049] (1) According to the molar ratio of 0.5:15, the solid FeCl 3 ·6H 2 O was dissolved in distilled water, and magnetically stirred for 3 minutes at room temperature to fully dissolve it to obtain solution a.
[0050] (2) Slowly add the triethanolamine (TEA) solution to solution a, the volume ratio of triethanolamine (TEA) to distilled water is 10:1, continue magnetic stirring for 1 minute to obtain brown-red solution b.
[0051] (3) Adjust the pH of the solution b to 8 with ammonia water to obtain a dark brown solution c.
[0052] (4) Put solution c into the reaction kettle, react at 160℃ for 6 hours, take out the product by magnetic separation method, and dry the obtained product in an oven at 70℃ for 2 hours to obtain superparamagnetic Fe 3 O 4 nanomaterials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com