Amantadine nitrate compound with neuroprotective effect, and preparation therefor and medical application thereof
An amantadine nitrate, neuroprotective technology, applied in the field of medicine, can solve the problems of not being able to fundamentally cure diseases, blocking the process of neurodegeneration and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
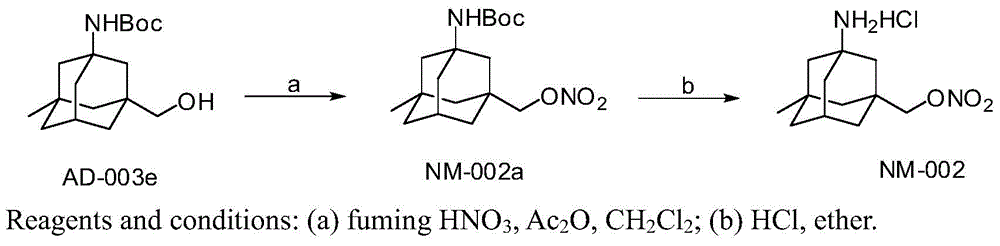
[0057] Embodiment 1, the synthesis of compound NM-002a
[0058] Compound AD-003e1.48g (5mmol) was dissolved in 30mL of dry dichloromethane and cooled in an ice-water bath. Add 3 mL of a mixture of acetic anhydride and fuming nitric acid (the volume ratio of acetic anhydride: fuming nitric acid is equal to 3:2). Maintain an ice-water bath and react for 10-15 minutes. The reaction solution was poured into 30 mL of 1N sodium bicarbonate solution, and the dichloromethane was separated, and the aqueous layer was extracted with dichloromethane (20 mL×3). The dichloromethane was combined, washed with 30 mL of water, dried over anhydrous sodium sulfate, filtered, and the dichloromethane was distilled under reduced pressure to obtain a colorless oily crude product. Silica gel column separation (petroleum ether:dichloromethane=10:1) yielded 1.07 g (62.9%) of colorless oil NM-002a. ESI-MS: m / z340.2 ([M] + ). 1 H-NMR(DMSO-d6,ppm):0.83(s,3H),1.15-1.24(m,2H),1.26-1.47(m,14H),1.56-1.80(...
Embodiment 2
[0059] Embodiment 2, the synthesis of compound NM-002
[0060] To compound NM-002a 680mg (2mmol), add 5mL of hydrogen chloride-saturated ether solution, react at room temperature, and spot plate monitoring. At the end of the reaction, a white solid precipitated out. Filter and wash the white solid with anhydrous ether to obtain 390 mg (70.7%) of pure NM-002. ESI-MS: m / z341.0 ([M+H] + ). 1 H-NMR(DMSO-d6,ppm):0.88(s,3H),1.19-1.29(m,2H),1.30-1.38(m,2H),1.38-1.52(m,4H),1.54-1.64(m ,2H), 1.66-1.73(m,2H), 2.18-2.24(m,1H), 4.29(s,2H), 8.11(s,3H).
Embodiment 3
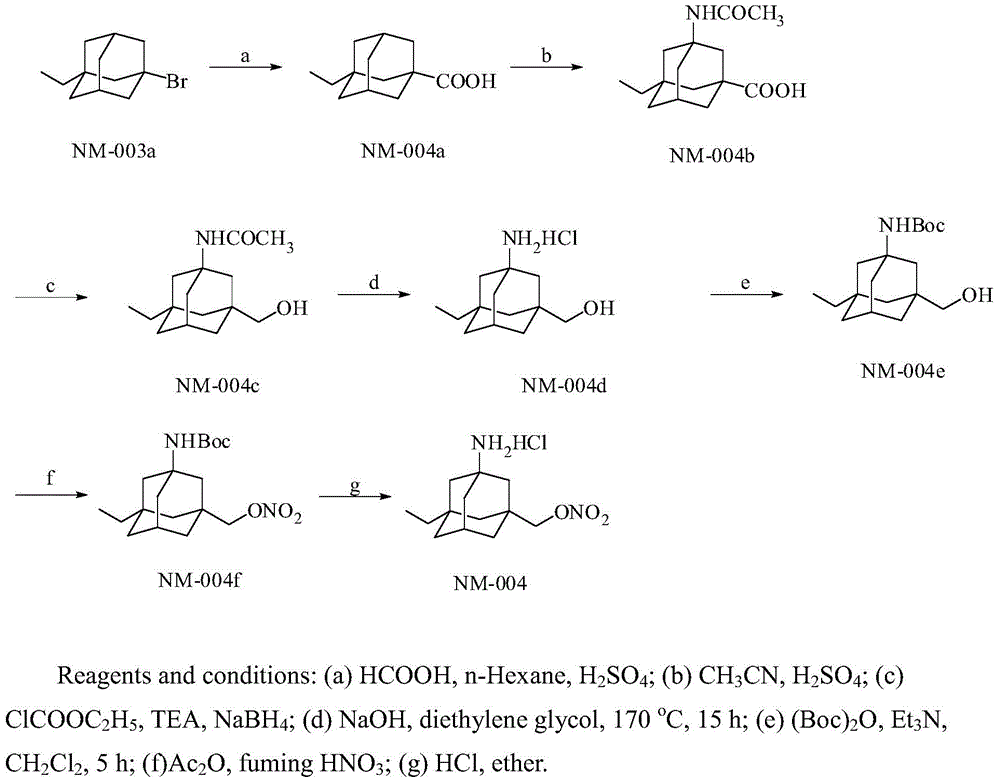
[0061] Embodiment 3, the synthesis of compound NM-004a
[0062] Take a 50mL round-bottom flask and place it in an ice-water bath to cool it, and add 20mL of concentrated sulfuric acid, 2mL of n-hexane and 970mg (4mmol) of compound NM-003a into the round-bottom flask. Maintaining the ice-water bath, formic acid (1.8 mL) was slowly added dropwise. After dropping, continue to react in ice-water bath for 3 hours. The reaction solution was poured into 100 mL of ice water, and a solid was precipitated. After standing still, a light yellow solid was obtained after suction filtration. After the solid was dried, it was dissolved in ethyl acetate, and the aqueous sodium hydroxide solution was basified to a pH of about 9-10, and the aqueous layer was separated. The organic layer was extracted with aqueous sodium hydroxide solution (30 mL×3), the aqueous solutions were combined, and the aqueous layer was acidified with dilute hydrochloric acid solution to a pH of about 3. Suction filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com