Porous polymer material used for preparing supercapacitor electrode and preparation method thereof
A technology of porous polymer and supercapacitor, applied in the field of material science, to achieve the effect of simple preparation method, improved specific capacitance and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The first step, the preparation of a novel dinitrile monomer containing a bisdiazinone structure
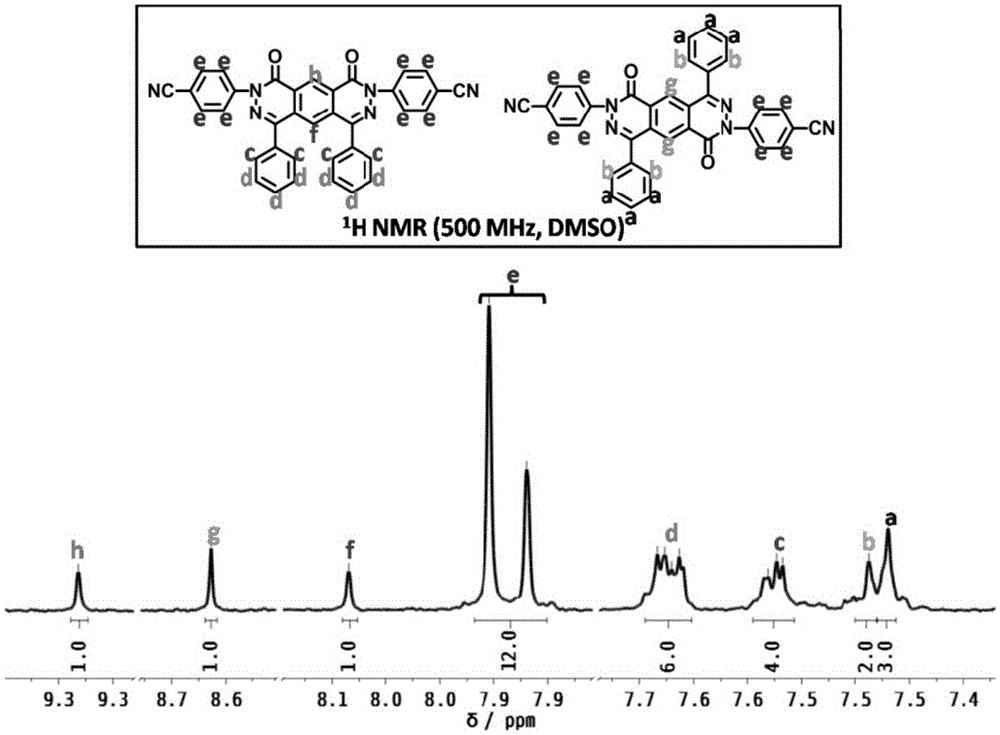
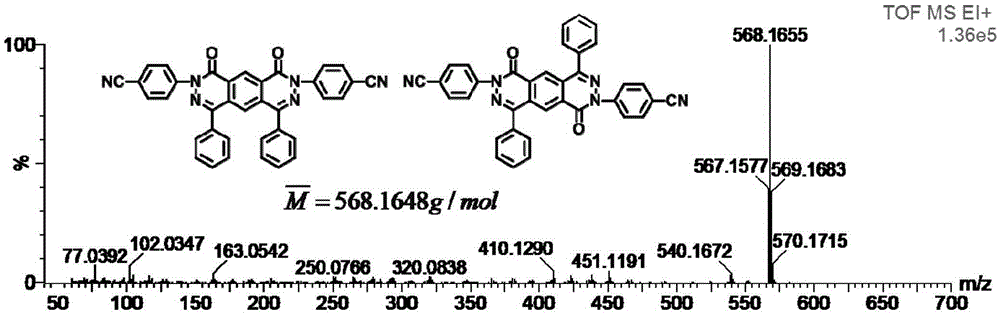
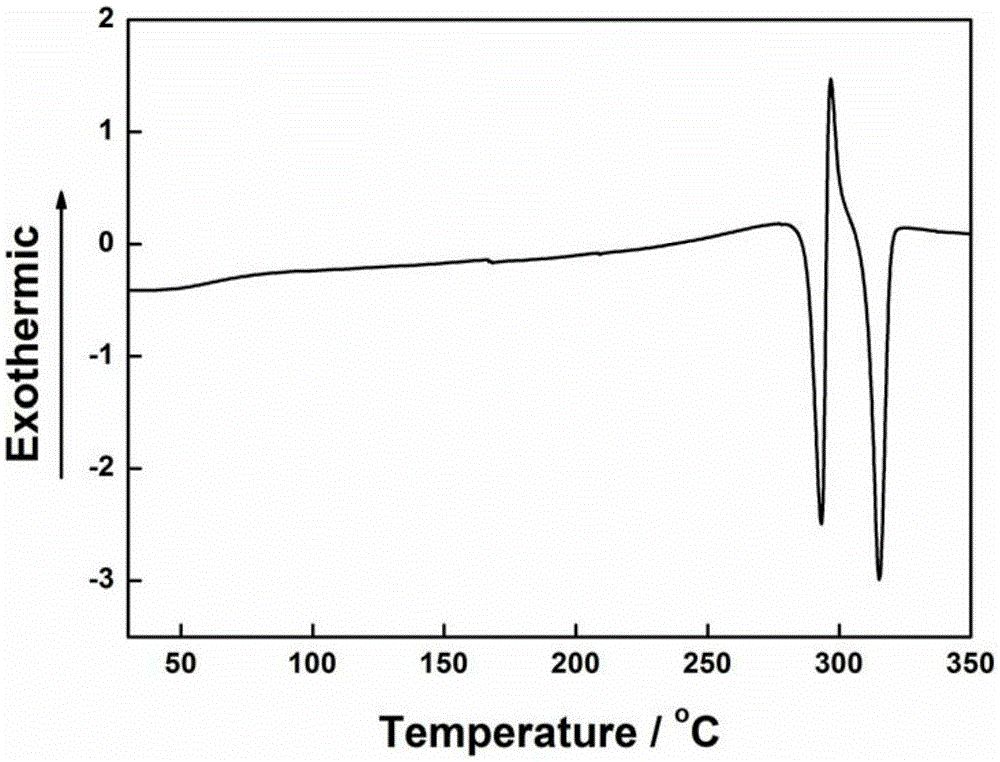
[0048] Add compound 1a (37g) (structural formula such as figure 1 Shown), 4-fluorobenzonitrile (30g), cesium fluoride (37g), then add 100ml of dimethyl sulfoxide DMSO to it, stir, react at 150°C for 5h, sink the solution into ethanol, Stand still, suction filter, Soxhlet extraction, and dry to obtain a yellow powdery solid. Product characterization as attached figure 1 ~ attached Figure 4 shown.
[0049] The second step, the preparation of porous polymer containing phthalazinone and s-triazine structure
[0050] Put the new dinitrile monomer (0.5g) containing bisdiazinone structure and anhydrous zinc chloride (0.2g) into a quartz tube, vacuum seal the tube, polymerize at 500°C for 30h, and cool the furnace to room temperature , take it out, put the obtained product in 5% dilute hydrochloric acid, ultrasonic 1-2h, centrifuge, wash with deionized water to neutrality, f...
Embodiment 2
[0056] The first step, the preparation of a novel dinitrile monomer containing a bisdiazinone structure
[0057] As described in Example 1.
[0058] The second step, the preparation of the polymer containing phthalazinone and s-triazine structure
[0059] The polymerization temperature was changed to 550° C., and other processing conditions were the same as in Example 1. Finally, a polymer polymerized at 550°C was obtained.
[0060] The specific Raman spectrum of the product obtained is shown in the appendix Figure 5 . Infrared spectrum see attached Figure 6 . Nitrogen adsorption and desorption curves and pore size distribution refer to the attached Figure 7 .
[0061] In order to further verify its electrical properties, electrochemical performance tests were carried out.
[0062] As described in Example 1.
[0063] The change of the specific capacity of the electrode material with the current density and the AC impedance spectrum can be found in the appendix Fi...
Embodiment 3
[0065] The first step, the preparation of a novel dinitrile monomer containing a bisdiazinone structure
[0066] As described in Example 1.
[0067] The second step, the preparation of the polymer containing phthalazinone and s-triazine structure
[0068] The polymerization temperature was changed to 600° C., and other processing conditions were the same as in Example 1. Finally, a polymer polymerized at 600°C was obtained.
[0069] The specific Raman spectrum of the product obtained is shown in the appendix Figure 5 . Infrared spectrum see attached Figure 6 . Nitrogen adsorption and desorption curves and pore size distribution refer to the attached Figure 7 . For the high-resolution TEM spectrum and the distribution labels of carbon atoms, nitrogen atoms, and oxygen atoms, please refer to the attached Figure 8 .
[0070] In order to further verify its electrical properties, electrochemical performance tests were carried out.
[0071] As described in Example 1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com