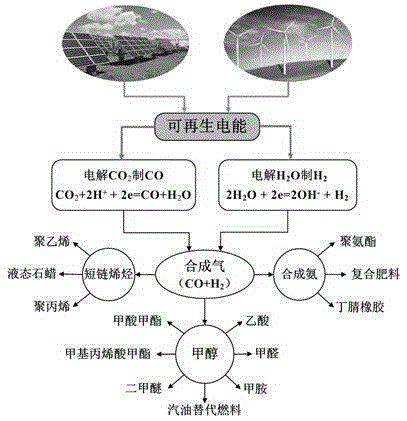
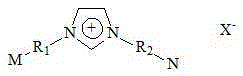Multi-room diaphragm electrolysis method and device for electroreduction of carbon dioxide into carbon monoxide
A carbon dioxide, carbon monoxide technology, applied in the electrolysis process, electrolysis components, cells, etc., can solve the problems of non-renewable fossil fuels, greenhouse gas effect, etc., achieve high electrochemical stability, promote migration, and reduce overpotential effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
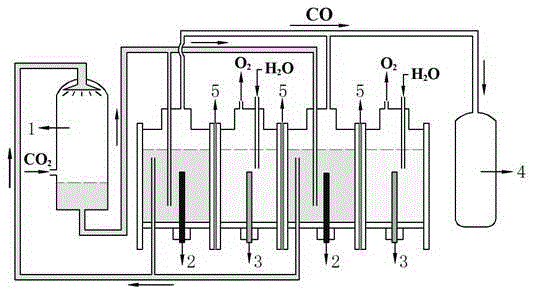
[0042] A multi-chamber diaphragm electrolysis method for electroreducing carbon dioxide to carbon monoxide: use a perfluorosulfonic acid ion exchange membrane to separate the electrolytic cell into multiple electrode chambers to form a multi-chamber diaphragm electrolytic cell in which the cathode chamber and the anode chamber are arranged alternately. The liquid is an organic composite electrolyte dissolved in carbon dioxide. The electrolyte in the anode chamber is an aqueous solution containing a supporting electrolyte. The cathode adopts a metal electrode with high selectivity for the electrolysis of carbon dioxide to produce carbon monoxide. The anode is an inert electrode. During the electrolysis reaction, there is a Carbon monoxide is produced, and hydrogen is produced as a by-product.
[0043] The organic composite electrolyte contains four functional components: an organic solvent, an organic supporting electrolyte, a proton conduction enhancer and an electrocatalyst. ...
Embodiment 2
[0052] A multi-chamber diaphragm electrolysis method for electroreducing carbon dioxide to carbon monoxide: use a perfluorosulfonic acid ion exchange membrane to separate the electrolytic cell into multiple electrode chambers to form a multi-chamber diaphragm electrolytic cell in which the cathode chamber and the anode chamber are arranged alternately. The liquid is an organic composite electrolyte dissolved in carbon dioxide. The electrolyte in the anode chamber is an aqueous solution containing a supporting electrolyte. The cathode adopts a metal electrode with high selectivity for the electrolysis of carbon dioxide to prepare carbon monoxide. The anode is an inert electrode. During the electrolysis reaction, there is a Carbon monoxide is produced, and hydrogen is produced as a by-product.
[0053] The organic composite electrolyte contains four functional components: an organic solvent, an organic supporting electrolyte, a proton conduction enhancer and an electrocatalyst. ...
Embodiment 3
[0062] A multi-chamber diaphragm electrolysis method for electroreducing carbon dioxide to carbon monoxide: use a perfluorosulfonic acid ion exchange membrane to separate the electrolytic cell into multiple electrode chambers to form a multi-chamber diaphragm electrolytic cell in which the cathode chamber and the anode chamber are arranged alternately. The liquid is an organic composite electrolyte dissolved in carbon dioxide. The electrolyte in the anode chamber is an aqueous solution containing a supporting electrolyte. The cathode adopts a metal electrode with high selectivity for the electrolysis of carbon dioxide to prepare carbon monoxide. The anode is an inert electrode. During the electrolysis reaction, there is a Carbon monoxide is produced, and hydrogen is produced as a by-product.
[0063] The organic composite electrolyte contains four functional components: an organic solvent, an organic supporting electrolyte, a proton conduction enhancer and an electrocatalyst. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com