7-ethyl-10-hydroxycamptothecine drug precursor, preparation method and application thereof
A technology of hydroxycamptothecin and ethyl, which is applied in the field of camptothecin drug precursors, can solve problems such as not being directly used in clinical practice, difficult quality control, cumbersome preparation process, etc., achieving good market prospects and clinical application value, The effect of high stability and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
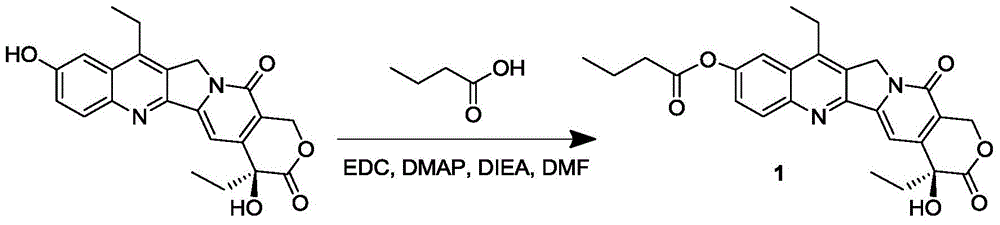
[0060] The synthesis of the SN-38 prodrug 1 of embodiment 1 containing alkane chain compound
[0061] Add n-butyric acid (116 μL, 1.3 mmol) and SN-38 (500 mg, 1.3 mmol) into a 100 mL round bottom flask, dissolve in 28 mL of anhydrous DMF (dimethylformamide), and then add EDC·HCl (267 mg, 1.4 mmol), DMAP (4-dimethylaminopyridine) (172 mg, 1.4 mmol) and DIEA (N,N-diisopropylethylamine) (232 μL, 1.4 mmol). Stir overnight at 25°C, remove the reaction solvent, dissolve the solid in dichloromethane, and then wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine; the organic phase is dried with anhydrous sodium sulfate, filtered, and the filtrate is collected to reduce The solvent was removed under pressure; the solid was separated and purified by column chromatography (DCM:MeOH=75:1) to obtain product 1 (350 mg, yield 59%).
[0062] product 1 1 HNMR nuclear magnetic data and mass spectrometry data are as follows:
[0063] 1 HNMR (400MHz, CDCl 3 ): δ1.02-1....
Embodiment 2
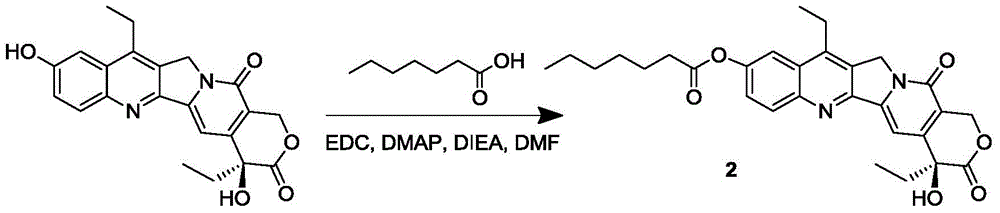
[0065] The synthesis of the SN-38 prodrug 2 of embodiment 2 containing alkane chain compound
[0066] Add n-heptanoic acid (108μL, 0.76mmol) and SN-38 (300mg, 0.76mmol) into a 100mL round bottom flask, dissolve in 20mL of anhydrous DMF, then add EDC·HCl (160mg, 0.84mmol), DMAP (103mg , 0.84mmol) and DIEA (149mL, 0.84mmol); stirred overnight at 25°C, after removing the reaction solvent, the solid was dissolved in dichloromethane, and then washed with 5% citric acid, saturated sodium bicarbonate, and saturated brine; the organic phase Dry over anhydrous sodium sulfate, filter, collect the filtrate and remove the solvent under reduced pressure; the solid is separated and purified by column chromatography (DCM:MeOH=75:1) to obtain product 2 (157 mg, yield 41%).
[0067] product 2 1 HNMR nuclear magnetic data and mass spectrometry data are as follows:
[0068] 1 HNMR (400MHz, CDCl 3 ): δ0.91-0.94(t,3H),1.00-1.04(t,3H),1.35-1.41(m,6H),1.43-1.49(t,3H),1.80-1.91(m,4H),2.64 -2.68(...
Embodiment 3
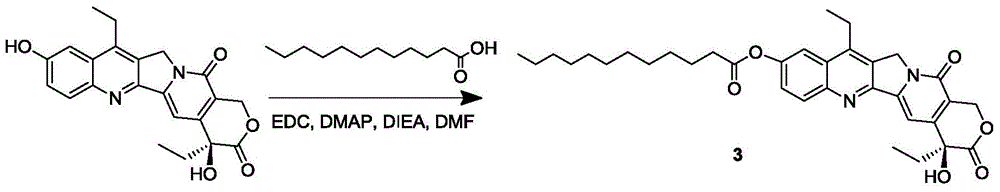
[0070] The synthesis of the SN-38 prodrug 3 of embodiment 3 containing alkane chain compound
[0071] Add dodecanoic acid (153mg, 0.76mmol) and SN-38 (300mg, 0.76mmol) in a 100mL round bottom flask, dissolve in 20mL of anhydrous DMF, then add EDC·HCl (160mg, 0.84mmol), DMAP (103mg , 0.84mmol) and DIEA (149μL, 0.84mmol); stirred overnight at 25°C, after removing the reaction solvent, the solid was dissolved in dichloromethane, and then washed with 5% citric acid, saturated sodium bicarbonate, and saturated brine; the organic phase Dry over anhydrous sodium sulfate, filter, collect the filtrate and remove the solvent under reduced pressure; the solid is separated and purified by column chromatography (DCM:MeOH=100:1) to obtain product 3 (220mg, 50%).
[0072] product 3 1 HNMR nuclear magnetic data and mass spectrometry data are as follows:
[0073] 1 HNMR (400MHz, CDCl 3 ): δ0.86-0.90(t,3H),1.01-1.05(t,3H),1.25-1.35(m,16H),1.40-1.43(t,3H),1.78-1.91(m,4H),2.64 -2.68(t,2H),3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com