Preparation method for montelukast sodium intermediate
A technology of intermediates and solids, which is applied in the field of preparation of Montelus-Turner intermediates, can solve the problems of separation waste and unusable chiral alcohol, etc., and achieve the effect of green and environmental protection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
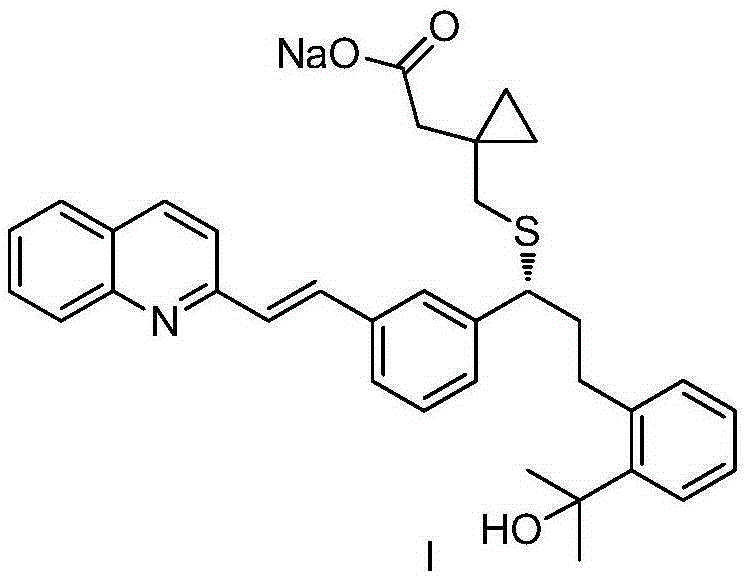
[0028] A kind of preparation method of montelukast sodium intermediate of the present invention comprises following reaction process:
[0029]
[0030] Described reaction process comprises the following steps:
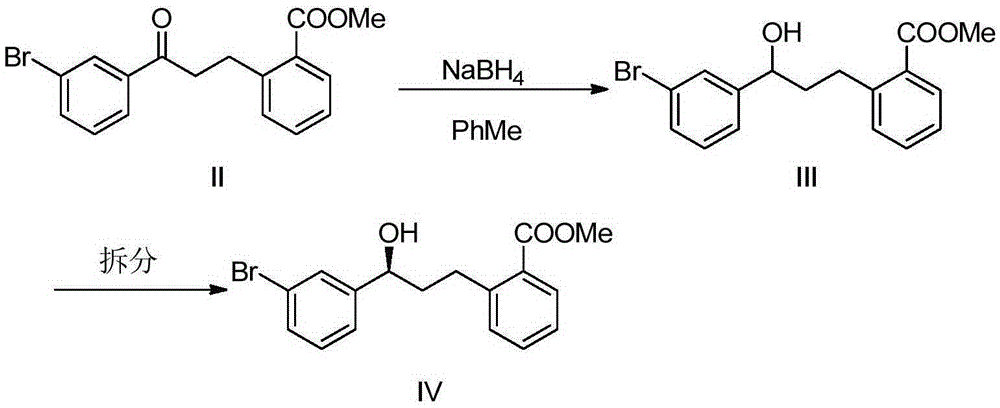
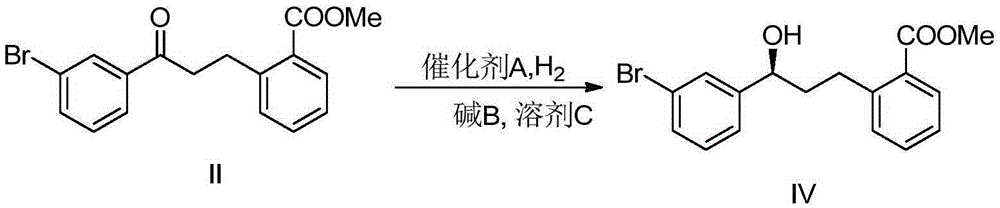
[0031] In the first step, the raw material methyl 2-(3-(3-bromophenyl)-3-oxopropyl)benzoate (II) is provided.
preparation example
[0033] The preparation method of catalyst A comprises the following steps:
[0034] (1) After dissolving p-xylene with concentrated sulfuric acid at 0°C, add concentrated nitric acid dropwise, first react at this temperature for 1 hour, then raise the temperature to 100°C and continue the reaction for 2 hours. After the reaction is complete, introduce the reaction system into crushed ice , continue to stir to obtain a yellow solid;
[0035] (2) The yellow solid obtained in the step (1) was catalyzed by 10% Pd / C, stirred for 4 h under a hydrogen pressure of 1 atm, filtered off 10% Pd / C, and the reaction solution was concentrated to obtain a brownish-yellow oil;
[0036] (3) After dissolving the oil obtained in step (2) in dichloromethane, add it dropwise to Cbz-L-amino acid and CDI at 0°C, stir at room temperature for 2 hours, add water to quench, and wash the water layer with dichloromethane Extracted with methane, combined with dichloromethane, concentrated to give a light g...
Embodiment 1
[0041] A preparation method of montelukast sodium intermediate, comprising the following steps:
[0042] At first, in 100L autoclave, under the condition of communicating with argon gas, add raw material reactant 10kg methyl 2-(3-(3-bromophenyl)-3-oxopropyl group) methyl benzoate ( II), then add 60kg of toluene as a solvent to fully dissolve the raw material (II), continue to feed argon for bubbling and degassing, and continue bubbling for 1.5 hours; the degassing is completed. Under an argon atmosphere, add 2.5g of catalyst (R,R)-DIOPRuCl2(R)-Me-BIMAH from the feeding port, and finally add 150g of potassium tert-butoxide. After the addition, quickly close the feeding port.
[0043] Secondly, replace the argon with hydrogen, slowly introduce hydrogen to 35 atmospheres, then close the inflation valve, close the hydrogen channel, and finally stir, keep the reaction at 35°C, and the pressure will drop after starting to stir, observe the change of pressure, When the pressure rema...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com