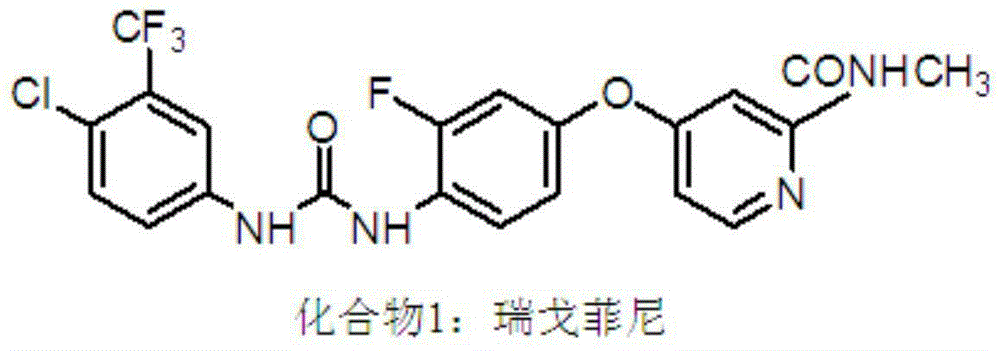
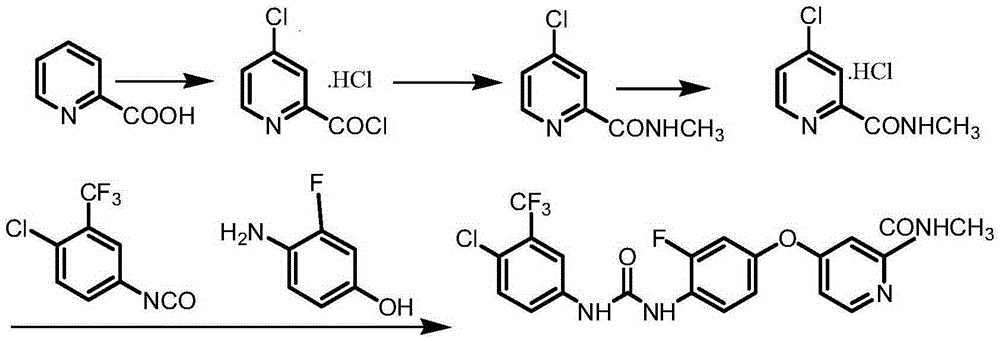
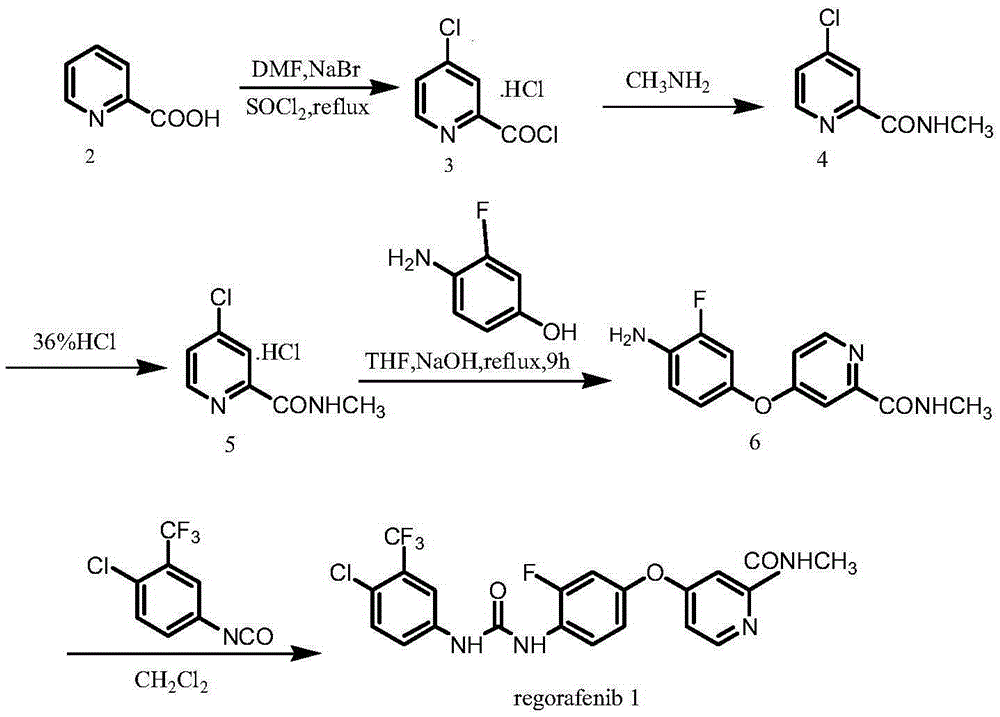
Preparation method for Regorafenib hydrate
A technology of regorafenib and compound, applied in the field of preparation of regorafenib, can solve the problems of unstable chemical properties of excipients, increased production cost, difficult post-processing, etc., and achieves a short cycle, convenient operation and few reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 4)
[0035] Sodium hydroxide aqueous solution (400mL, 2.5M) was added dropwise to 4-chloro-3-trifluoromethylaniline (compound 2) (78.2g, 400mmol) in 400ml ethyl acetate solution, the temperature was controlled below 5°C, and Stir at 0°C to 5°C for 30min; add propylene chloroformate (compound 3) (59.5ml, 560mmol) dropwise, and control the temperature of the dropwise addition process below 5°C; stir the reaction mixture at room temperature for 1-3 hours, separate the liquids, The aqueous phase was extracted with ethyl acetate (3×800mL), the combined organic phases were washed with water (3×1000mL), anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate to the crude product, add ethyl acetate:n-heptane (1:2) for recrystallization, filter with suction, and dry to obtain (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 4 ) 101.0g, yield 90.3%, purity 99.3% (HPLC meth...
Embodiment 2
[0036] Embodiment 2: Synthesis of (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 4)
[0037] Sodium hydroxide aqueous solution (100mL, 3.5M) was added dropwise into 100ml ethyl acetate solution of 4-chloro-3-trifluoromethylaniline (compound 2) (19.6g, 100mmol), the temperature was controlled below 5°C, and Stir at 0°C to 5°C for 30min; add propylene chloroformate (compound 3) (32ml, 300mmol) dropwise, and control the temperature of the dropping process below 5°C; stir the reaction mixture at room temperature for 1-3 hours, separate liquid, and phase was extracted with ethyl acetate (3×200mL), the organic phases were combined, washed with water (3×500mL), anhydrous Na 2 SO 4Dry, filter, and concentrate the filtrate to the crude product, add ethyl acetate:n-heptane (1:2) for recrystallization, filter with suction, and dry to obtain (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 4 ) 25.5g, yield 91.3%, purity 99.2% (HPLC method).
Embodiment 3
[0038] Example 3: Synthesis of (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 4)
[0039] Add sodium ethoxide (6.85g, 100mmol) and 4-chloro-3-trifluoromethylaniline (compound 2) (19.6g, 100mmol) into 100ml ethanol solution, control the temperature below 5°C, and Stir at ℃ for 30min; add propylene chloroformate (compound 3) (11ml, 100mmol) dropwise, and control the dropwise addition process temperature below 5℃; the reaction mixture is stirred at room temperature for 1-3 hours, concentrated, and extracted with ethyl acetate (3 ×200mL), combined the organic phases, washed with water (3×500mL), anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate to the crude product, add ethyl acetate:n-heptane (1:2) for recrystallization, filter with suction, and dry to obtain (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 4 ) 25.7g, yield 92.0%, purity 99.4% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com