A HRM detection method and primers for rapid identification of mouse encephalomyelitis virus and rat Theileria virus
An encephalomyelitis virus, rat Taylor virus technology, applied in biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragment, etc. problem, to achieve the effect of fast detection speed, good repeatability, and high throughput of detection speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The design of embodiment 1 specific primer
[0059] The present invention designs the primers that can detect TMEV and RTV simultaneously; And a large amount of experimental screenings are done to the designed primers, and a pair of strong specificity primers are screened out, and its nucleotide sequence is as follows:
[0060] Upstream primer P1: ATTTGAAAGCAATGGTTAGC (SEQ ID NO: 1);
[0061] Downstream primer P2: GATCGAGAGGATGTTCATCTAA (SEQ ID NO: 2).
Embodiment 2
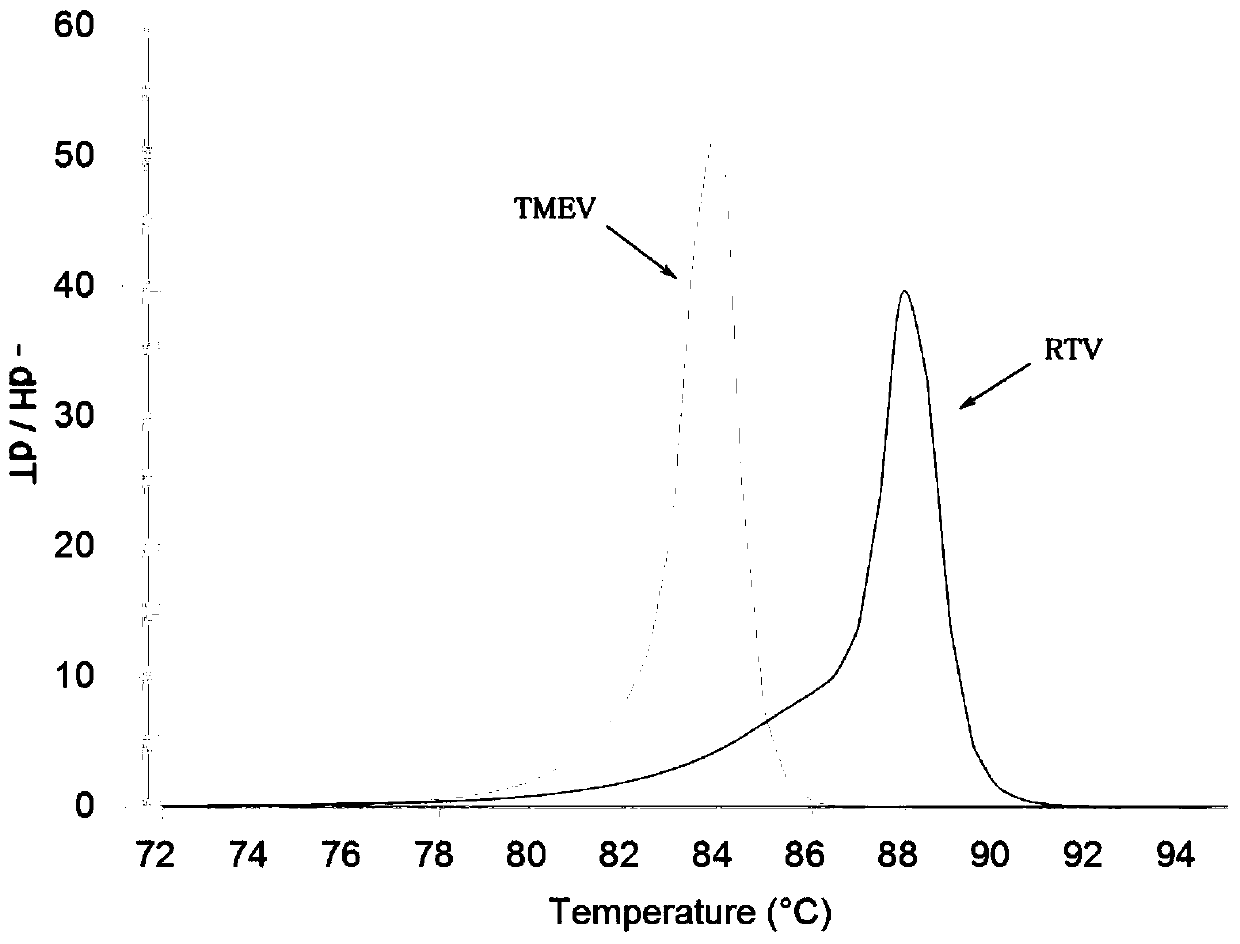
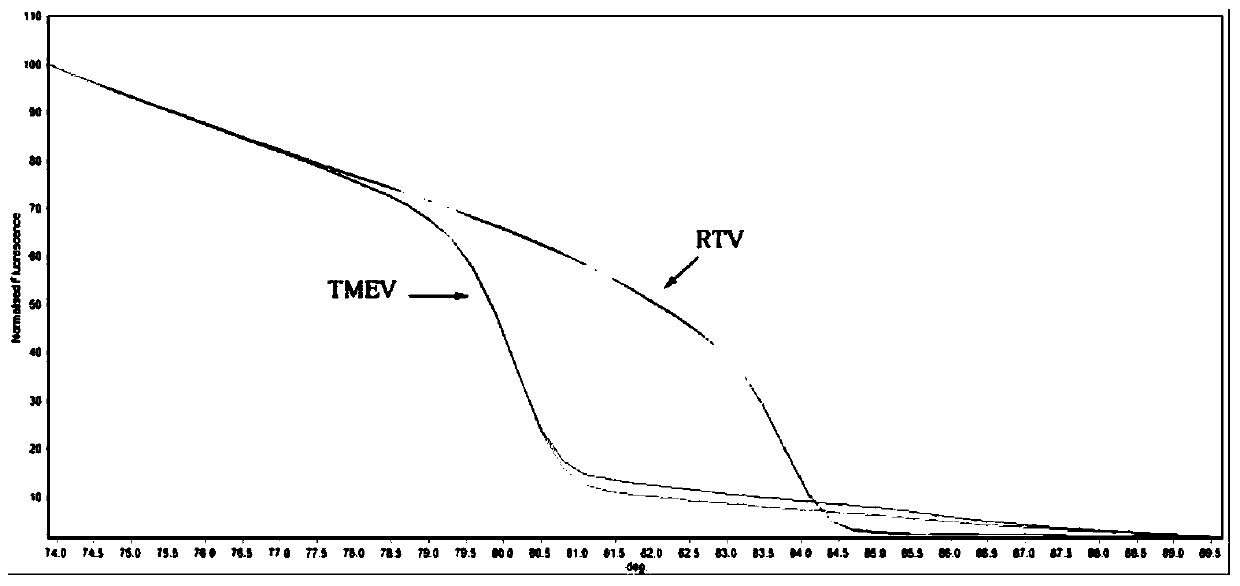
[0062] The PCR-HRM analysis of embodiment 2 plasmid standards
[0063] (1) Preparation of TMEV and RTV plasmid standards:
[0064] The RNAs of mouse encephalomyelitis virus and rat Theilera virus were extracted respectively, and primers P1 and P2 were used for RT-PCR amplification with PrimeScript One Step RT-PCR Kit Ver.2 kit (Takara Company):
[0065] The reaction system is: Enzyme Mix 2 μl, 2×RT-PCR Buffer 25 μl, upstream primer P1 (10 μM ) 2.5 μl, downstream primer P2 (10 μM) 2.5 μl, RNA 5 μl, add RNase Free ddH 2 0 to 50 μl.
[0066] The reaction conditions were: reverse transcription at 50°C for 30 min, 94°C for 2 min, a cycle of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, a total of 35 cycles, and a final extension at 72°C for 7 min.
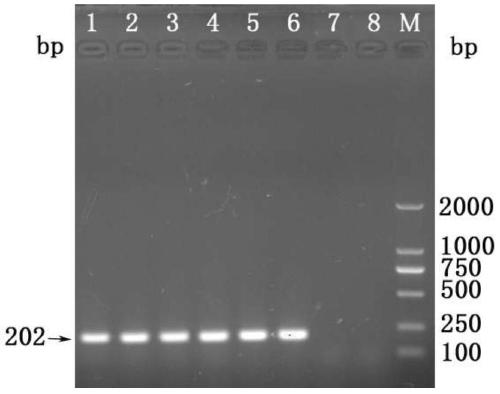
[0067] The PCR products were subjected to 2% agarose gel electrophoresis, and the target fragments were recovered using a gel recovery kit (Tiangen Company) and then cloned into the pGEM-T Easy vector (Promega Company) to obt...
Embodiment 3
[0080] Example 3 PCR-HRM detection of clinical samples
[0081] (1) Extraction of RNA in the sample to be tested:
[0082] The samples to be tested in the present invention are the contents of spleen and cecum of rats collected in Guangdong area, and TMEV and RTV genomic RNA in the samples are extracted with Trizol reagent (Invitrogen Company).
[0083] (2) One-step RT-PCR amplification:
[0084] The extracted genomic RNA was amplified by one-step RT-PCR using PrimeScript one step RT-PCR Kit Ver.2 kit.
[0085] The reaction system is as follows:
[0086]
[0087] The one-step RT-PCR amplification reaction procedure is as follows:
[0088] 50°C for 30 min; 94°C pre-denaturation for 3 min; 94°C denaturation for 20 sec, 55°C annealing for 20 sec, 72°C extension for 20 sec, 40 cycles; 72°C final extension for 5 min; the melting rate set for HRM analysis was 0.3°C / sec.
[0089] (3) Two-step amplification:
[0090] cDNA was obtained by reverse transcription using the RNA ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com