Fluorine-containing phenanthridine derivative and preparation method thereof
A technology containing fluorophenanthridine and derivatives, which is applied in the field of fluorophenanthridine derivatives and their preparation, can solve the problems of difluoromethylene/cyclization and the like that have not yet been seen, and achieves high yield and substrate adaptability. Wide range of effects and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
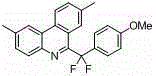
[0017] Preparation of 6-(difluoro(4-methoxyphenyl)methyl)phenanthridine adopts the following steps: in a 50 ml reaction flask, add
[0018] Add 0.102 g of silver nitrate, 1.37 g of ammonium persulfate, 0.15 g of potassium bicarbonate, 1.35 g of 2-isocyano-1,1'-biphenyl, 0.72 g of 2,2-difluoro-2-(4-methoxy Phenyl) potassium acetate, then add 25 ml of dimethyl sulfoxide, nitrogen protection, stir at 70 degrees for 8 hours, cool to room temperature after the reaction, remove the solvent, separate by silica gel column chromatography, the eluent is 10:1 A mixed solvent of petroleum ether and ethyl acetate was used to obtain 0.86 g of light yellow solid, with a yield of 86%.
[0019] 6-(difluoro(4-methoxyphenyl)methyl)phenanthridine, characterized in that the compound has the structure:
[0020]
[0021] Molecular formula: C 21 h 15 f 2 NO
[0022] Chinese name: 6-(difluoro(4-methoxyphenyl)methyl)phenanthridine
[0023] English name: 6-(difluoro(4-methoxyphenyl)methyl)phena...
Embodiment 2
[0032] Preparation of 6-(difluoro(4-methoxyphenyl)methyl)phenanthridine adopts the following steps: in a 500 ml reaction flask, add
[0033] Add 1.01 g of silver nitrate, 13.7 g of ammonium persulfate, 1.5 g of potassium bicarbonate, 13.5 g of 2-isocyano-1,1'-biphenyl, 7.2 g of 2,2-difluoro-2-(4-methoxy Phenyl) potassium acetate, then add 250 ml of dimethyl sulfoxide, nitrogen protection, stir at 80 degrees for 10 hours, cool to room temperature after the reaction, remove the solvent, and separate with silica gel column chromatography, the eluent is 10:1 The mixed solvent of sherwood oil and ethyl acetate obtained 8.844 grams of light yellow solid, yield 88%.
Embodiment 3
[0035] The following steps are used to prepare 6-(difluoro(4-methoxyphenyl)methyl)phenanthridine: in a 1-liter reaction flask, add
[0036] Add 10.1 grams of silver nitrate, 137 grams of ammonium persulfate, 15 grams of potassium bicarbonate, 135 grams of 2-isocyano-1,1'-biphenyl, 72 grams of 2,2-difluoro-2-(4-methoxy Phenyl) potassium acetate, then add 500 ml of dimethyl sulfoxide, nitrogen protection, stir at 80 degrees for 12 hours, cool to room temperature after the reaction, remove the solvent, separate by silica gel column chromatography, the eluent is 10:1 The mixed solvent of sherwood oil and ethyl acetate obtained 82.41 grams of light yellow solid, yield 82%.
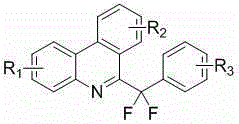
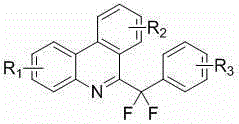
[0037] According to the above-mentioned process route for preparing 6-(difluoro(4-methoxyphenyl)methyl)phenanthridine, the preparation method and structural characterization of other related compounds protected by this patent application are as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com