Active-energy-ray-polymerizable resin composition and laminate
A resin composition and active energy ray technology, which is applied in the direction of synthetic resin layered products, epoxy resin glue, epoxy resin coating, etc., can solve the problems of large hardening shrinkage of adhesives, unevenness, and wrinkles of polarizers, etc. Achieve excellent durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0294] Hereinafter, specific examples of the present invention will be described together with comparative examples, but the present invention is not limited to the following examples. In addition, in the following examples and comparative examples, "parts" and "%" represent "parts by weight" and "% by weight", respectively.
[0295]In addition, "weight average molecular weight (Mw)" and "number average molecular weight (Mn)" are values measured using a gel permeation chromatograph "HLC-8220GPC" manufactured by Tosoh Co., Ltd., and a separation column: Tosoh Four "TSK-GELSUPERH5000", "TSK-GELSUPERH4000", "TSK-GELSUPERH3000", and "TSK-GELSUPERH2000" manufactured by Cao Co., Ltd. were connected in series, and tetrahydrofuran at a temperature of 40°C was used for the mobile phase at a rate of 0.6ml / min. The polystyrene-equivalent weight average molecular weight and the number average molecular weight measured at the flow rate of .
[0296] In addition, in this specification, c...
Synthetic example 1
[0337]
[0338] Add polytetramethylene glycol (manufactured by Hodogaya Chemical Co., Ltd.: PTG850, hydroxyl value 127.1 mgKOH / g) 81.6 parts and 41.4 parts of isophorone diisocyanate were heated up to 60 degreeC, introducing dry air. 0.05 part of dibutyltin dilaurate was added thereto, and reaction was performed for 1 hour. Separately, 27.0 parts of 4-hydroxybutyl acrylate and 0.15 parts of hydroquinone monomethyl ether were put into the dropping funnel, and were added dropwise to the separate flask over 1 hour. After completion of the dropwise addition, stirring was continued at 80° C. for 3 hours, and the reaction was terminated when no absorption peak of the isocyanate group was confirmed in the infrared absorption spectrum, and an oligomer 2 was obtained. The weight average molecular weight of the product was 4000.
[0339] In addition, the measuring method of the hydroxyl value of this invention is as follows. Accurately measure about 1 g of a sample in a stoppered E...
Synthetic example 2
[0346]
[0347] A compound obtained by adding 2 mol of ε-caprolactone to 1 mol of 2-hydroxyethyl acrylate (Dacel Chemical production: Placcel FA2D, hydroxyl value 163.0mgKOH / g) 113.4 parts, isophorone diisocyanate 36.6 parts, hydroquinone monomethyl ether 0.15 parts, while introducing dry air while raising the temperature to 60 ℃. 0.04 part of dibutyltin dilaurate was added thereto, the temperature was raised, and reaction was performed at 80° C. for 3 hours. The reaction was terminated when no isocyanate group absorption peak was confirmed in the infrared absorption spectrum. The weight average molecular weight of the product was 1300. In addition, the measurement of a hydroxyl value is the same as that of the synthesis example 1 of an oligomer.
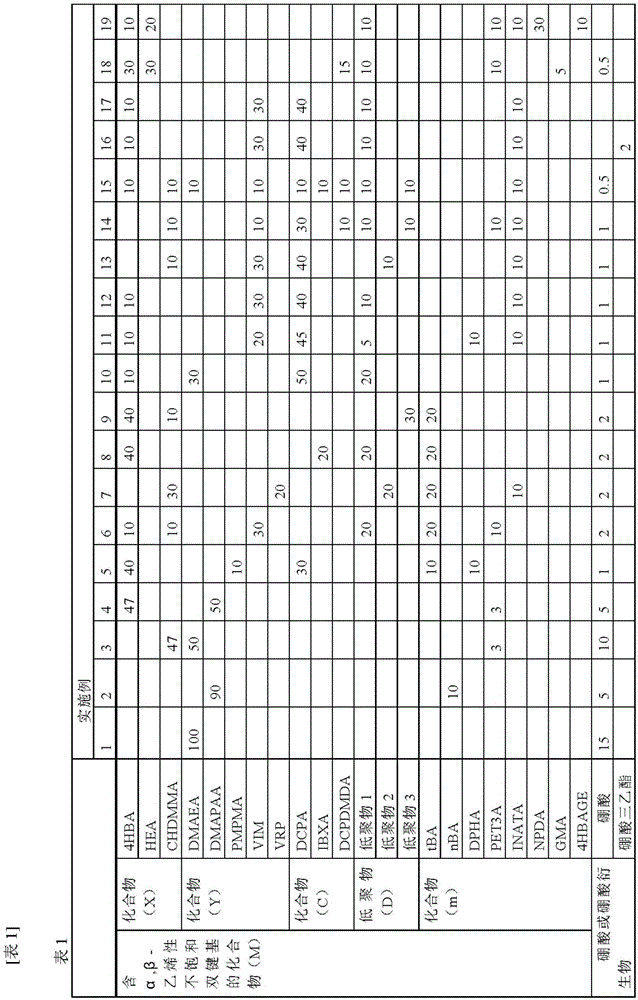
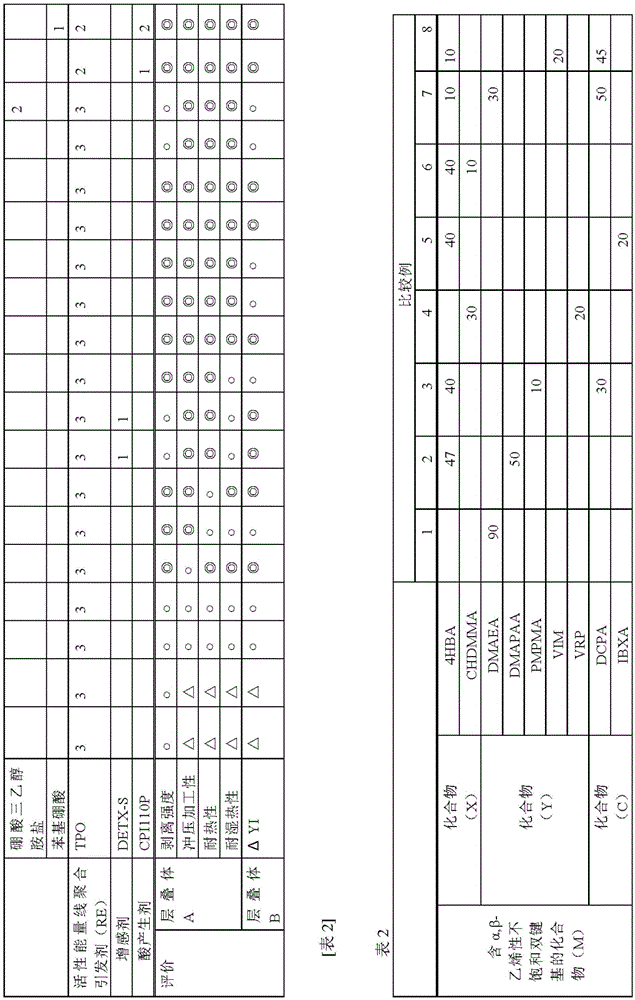
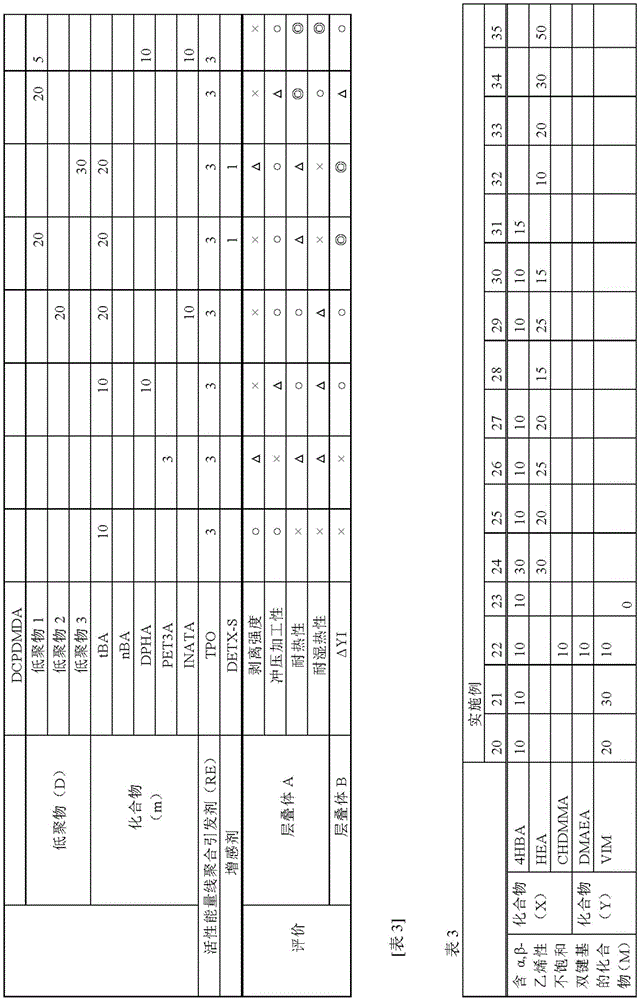
[0348] Using the active energy ray polymerizable adhesives prepared in the ratios of Tables 1 to 5, laminates were produced by the following methods, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com