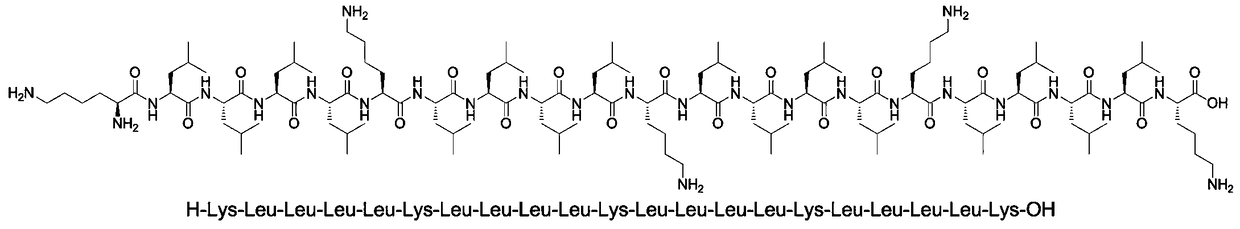
A kind of solid-liquid phase combination prepares the method for cinapultide
A technology of cinaputide and solid-liquid phase, which is applied in the field of solid-liquid phase preparation of cinaputide, which can solve the problems of long production cycle, low purity of crude product and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of Boc-Leu-OSu
[0060] Accurately weigh 1156g (5.0mol) of Boc-Leu-OH and 633g (5.5mol) of HOSu, dissolve them in 12L of tetrahydrofuran, and stir in an ice-water bath. Accurately weigh 1238g (6.0mol) of DCC, dissolve it in 7L tetrahydrofuran, slowly add it dropwise to the above solution, stir the reaction, and monitor the end point of the reaction by TLC. After the reaction was completed, filter with suction, concentrate the reaction solution to 6-7L, add 35L of petroleum ether to the concentrated solution, a large amount of white solid precipitated out, and the solution stood at -20°C for 1 hour. After standing still, filter with suction, dissolve the filter cake with 6L of ethyl acetate, add 35L of petroleum ether, crystallize, filter with suction, and dry the filter cake in vacuum to obtain 1538g of Boc-Leu-OSu with a purity greater than 99% and a yield of 93.7%.
Embodiment 2
[0061] Embodiment 2: Preparation of H-Leu-Leu-OH
[0062] Accurately weigh 511g (3.9mol) of H-Leu-OH and 615g (5.8mol) of sodium carbonate and dissolve them in 7L of water, and slowly add 8L of tetrahydrofuran solution of 1538g (4.7mol) of Boc-Leu-OSu obtained in Example 1 under an ice-water bath , the reaction was stirred, and the end point of the reaction was monitored by TLC. After the reaction is complete, filter, add 10% citric acid aqueous solution to the filtrate under an ice-water bath, adjust the pH value of the solution to 2-3, extract with 10L ethyl acetate, combine the organic phases, concentrate to 5-6L by rotary evaporation, and add to the concentrated solution Add 30L of petroleum ether, place it at -20°C to crystallize to obtain Boc-Leu-Leu-OH, filter it and suck it dry, dissolve it with 60% TFA / water solution, stir and react for 2 hours, wash it with cold ether, and dry it in vacuo to obtain H-Leu-Leu-OH 883g, purity greater than 99%, yield 92.7%.
Embodiment 3
[0063] Embodiment 3: Preparation of Fmoc-Leu-OSu
[0064]Accurately weigh 1767g (5.0mol) of Fmoc-Leu-OH and 633g (5.5mol) of HOSu, dissolve them in 14L of tetrahydrofuran, and stir in an ice-water bath. Accurately weigh 1238g (6.0mol) of DCC, dissolve it in 7L tetrahydrofuran, slowly add it dropwise to the above solution, stir the reaction, and monitor the end point of the reaction by TLC. After the reaction was completed, filter with suction, concentrate the reaction solution to 6-7L, add 35L of petroleum ether to the concentrated solution, a large amount of white solid precipitated out, and the solution stood at -20°C for 1 hour. After standing still, filter with suction, dissolve the filter cake with 6L of ethyl acetate, add 35L of petroleum ether, crystallize, filter with suction, and dry the filter cake in vacuum to obtain 2020g of Fmoc-Leu-OSu with a purity greater than 99% and a yield of 89.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com