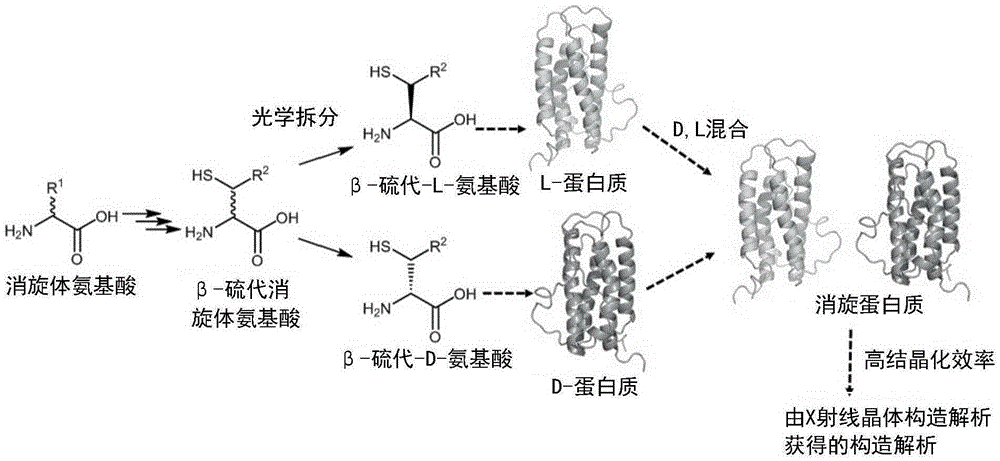
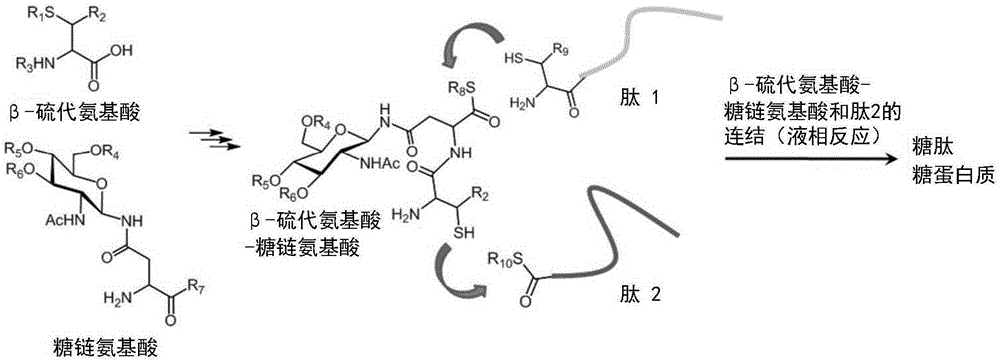
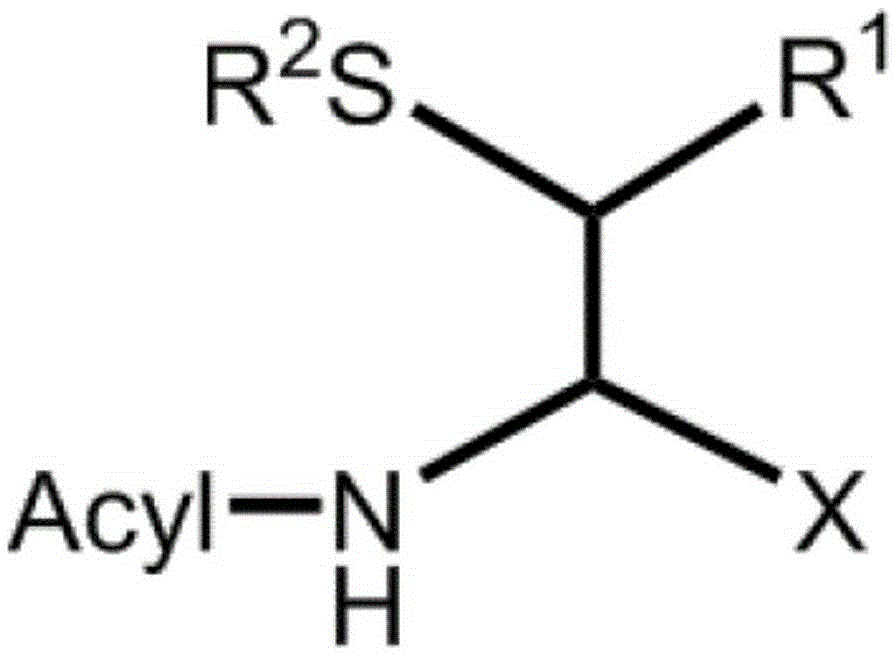
Method for producing d-form or l-form amino acid derivative having thiol group
A manufacturing method, amino acid technology, applied in the preparation of organic compounds, biochemical equipment and methods, preparation of thiols, etc., can solve the problems such as not specifically mentioned manufacturing, not reaching the level of manufacturing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0127] As the protecting group for the amino group, those generally used for the protecting group of the amino group can be used, for example, a fat-soluble protecting group described later can be used. In one embodiment of the present invention, for example, 9-fluorenylmethoxycarbonyl (Fmoc) or tertiary butoxycarbonyl (Boc), allyloxycarbonate (Alloc) and other carbonate-containing group, acyl group such as acetyl group (Ac), protective group such as aryl group, benzyl group, etc. To introduce a protecting group, for example, to introduce a Boc group, add Boc to the reaction system 2 O THF solution method and so on. The introduction of the protecting group of the amino group can be carried out by a known method other than the above-mentioned method depending on the protecting group. In addition, the deprotection of the protecting group of the amino group can be carried out by treating with an acid or a base. For example, when the protecting group is a Boc group, an acid suc...
Embodiment
[0219] Synthesis of Optically Active Aromatic Amino Acid Derivatives with a Thiol Group at the β Position Using Aromatic Amino Acids as Starting Materials
[0220] As shown in the following reaction formula, a synthesis reaction of D,L-phenylalanine having a thiol group at the β-position is carried out using an aromatic amino acid as a starting material. The compound numbers described after the compound names in the synthesis examples represent the compound numbers described in the following reaction formulas.
[0221]
Synthetic example 1
[0222] (Synthesis Example 1) D,L-Phenylalanine Methyl Ester Hydrochloride (Compound 6)
[0223] Under argon, D,L-phenylalanine (10.0 g, 60.5 mmol) was added to methanol (48.4 mL), and after the solution was cooled to 0° C., sulfinyl chloride (4.8 ml, 66.6 mmol) was added dropwise. Afterwards, it was stirred at reflux for 1 hour. After the reaction solution was concentrated under reduced pressure, recrystallization was performed using a mixed solvent of 5 ml of methanol and 80 mL of diethyl ether to obtain D,L-phenylalanine methyl ester hydrochloride (compound 6) (11.8 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com