Metformin chitosan microcapsule/starch composite film and preparation method thereof
A technology of chitosan microcapsule and starch composite film, which is applied in the field of biomedicine, can solve the problems of poor microcapsule encapsulation rate, lack of temperature sensitivity or pH sensitivity, poor water solubility of chitosan, etc., and achieve enhanced stability and solidification Better effect, not easy to swell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of preparation method of biguanide chitosan microcapsule / starch composite film, comprises the following steps:
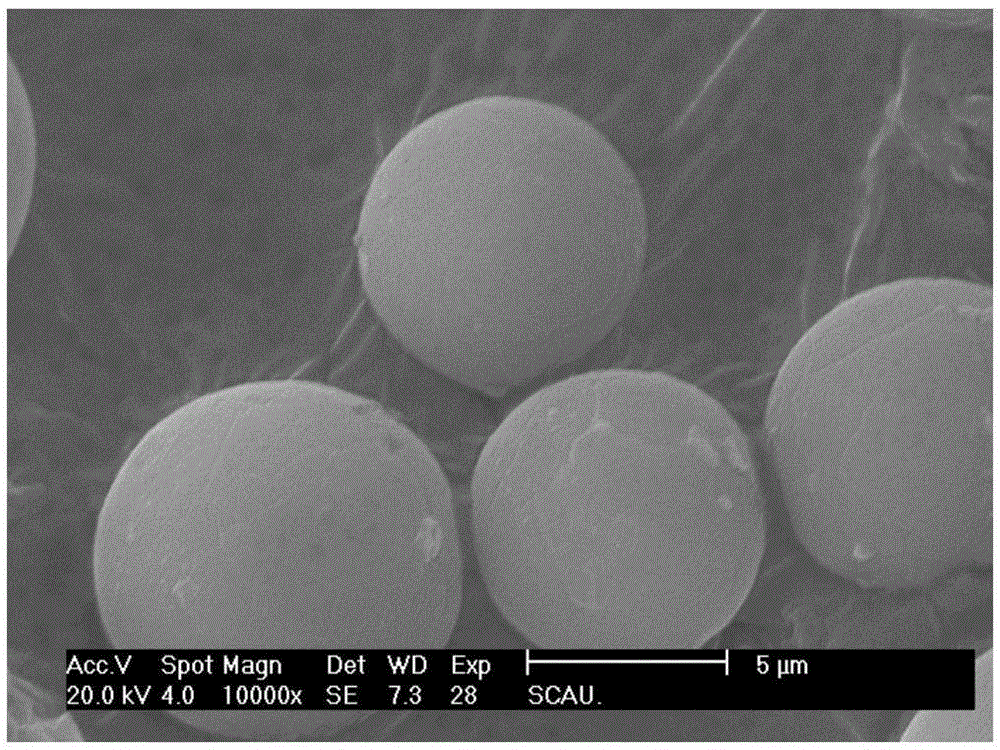
[0035](1) Preparation of biguanide microcapsules: Weigh 0.30 g of water-soluble drug and dissolve in 30 mL of biguanide chitosan-acetic acid solution with a concentration of 0.01 g / mL and stir to form a uniform aqueous phase; take 10 mL of Span 80 and Tween 20 and add into the 90mL solution mixed with petroleum ether and liquid paraffin at a volume ratio of 1:1 and stirred until uniform to obtain an oil phase; under shear stirring at a speed of 900rpm, add the water phase drop by drop to the oil phase, and add The time is 15min, and emulsified for 15min; 20mL glutaraldehyde is added dropwise for solidification, the dropping time is 1h, and the solidification time is 1h; washing with acetone, and drying in an oven at 40°C to obtain drug-loaded microcapsules with a particle size of 1-10 μm and an encapsulation efficiency of 85.70%.
[0036] (2) Composit...
Embodiment 2
[0042] (1) The difference between step (1) in this example and step (1) in Example 1 is that the addition of water-soluble drug is adjusted to 0.20 g, and the addition of glutaraldehyde is adjusted to 20 mL. Other steps and parameters were the same as step (1) in Example 1, and the encapsulation efficiency of the prepared drug-loaded microcapsules was 72.66%.
[0043] (2) With step (2) in embodiment 1.
Embodiment 3
[0045] (1) With step (1) in embodiment 2.
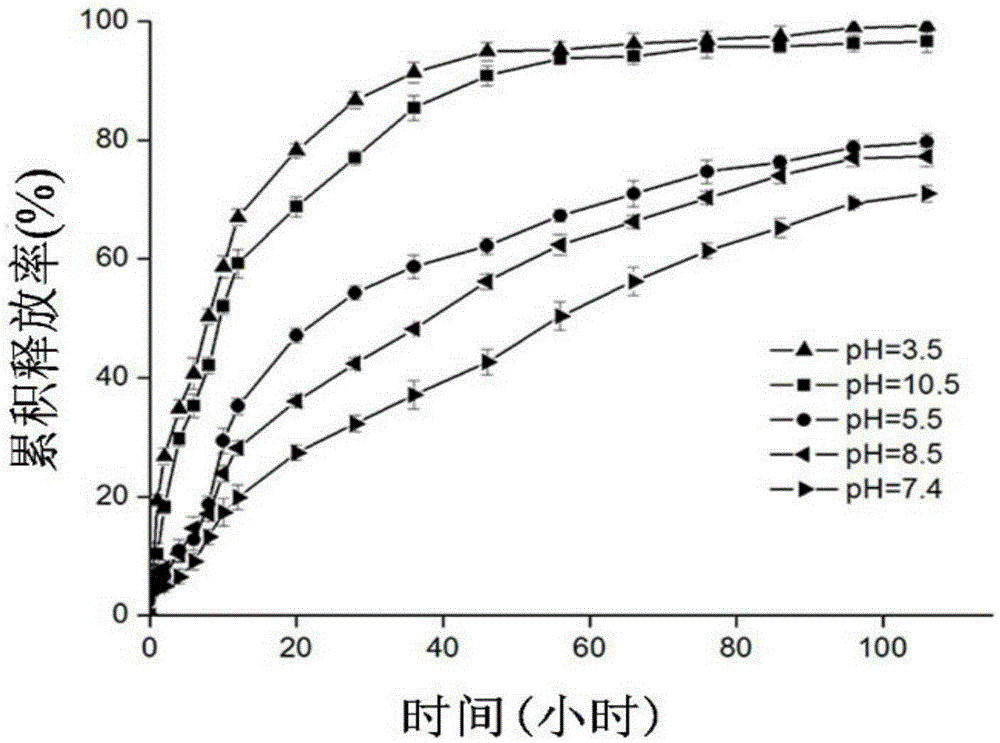
[0046] (2) The difference between step (2) in this example and step (2) in Example 1 is that the addition of agar powder is adjusted to 0.25 g, and the addition of drug-loaded microcapsules is adjusted to 0.010 g. Other steps and parameter are identical with step (1) in embodiment 1, and the composite film tensile strength that is prepared is 8.11Mpa, elongation at break is 80.36%, the biguanide chitosan microcapsule that drug-loaded microcapsule content is 1% / starch composite film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com