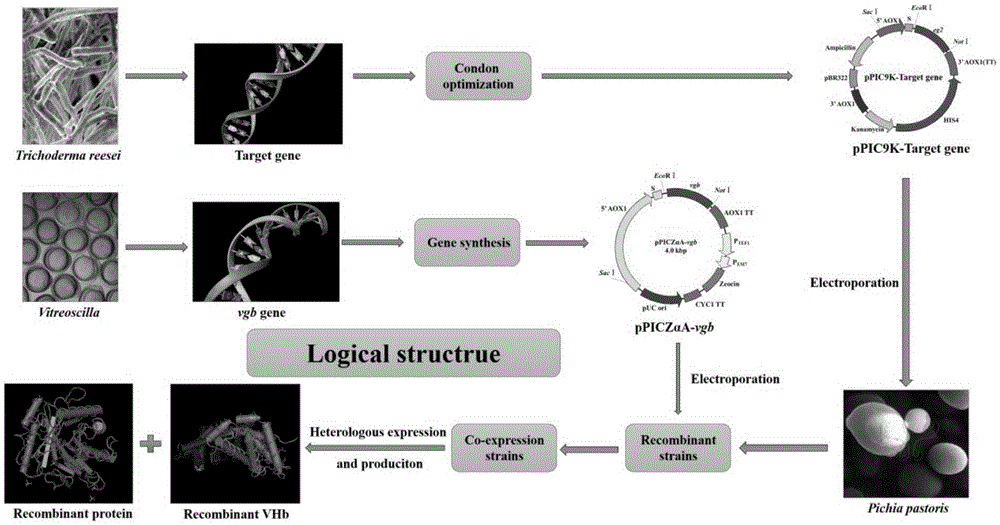
Construction method for co-expression hemoglobin VHb and cellulase protein in pichia pastoris
A Pichia pastoris and hemoglobin technology, applied in the field of recombinant Pichia pastoris system construction, can solve the problems of low enzymatic hydrolysis efficiency, lack of exonuclease, core enzyme components, etc. The effect of industrial production potential, improving enzyme production efficiency, and improving expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Trichoderma reesei endoglucanase II (EGII) gene codon optimization and expression vector construction.
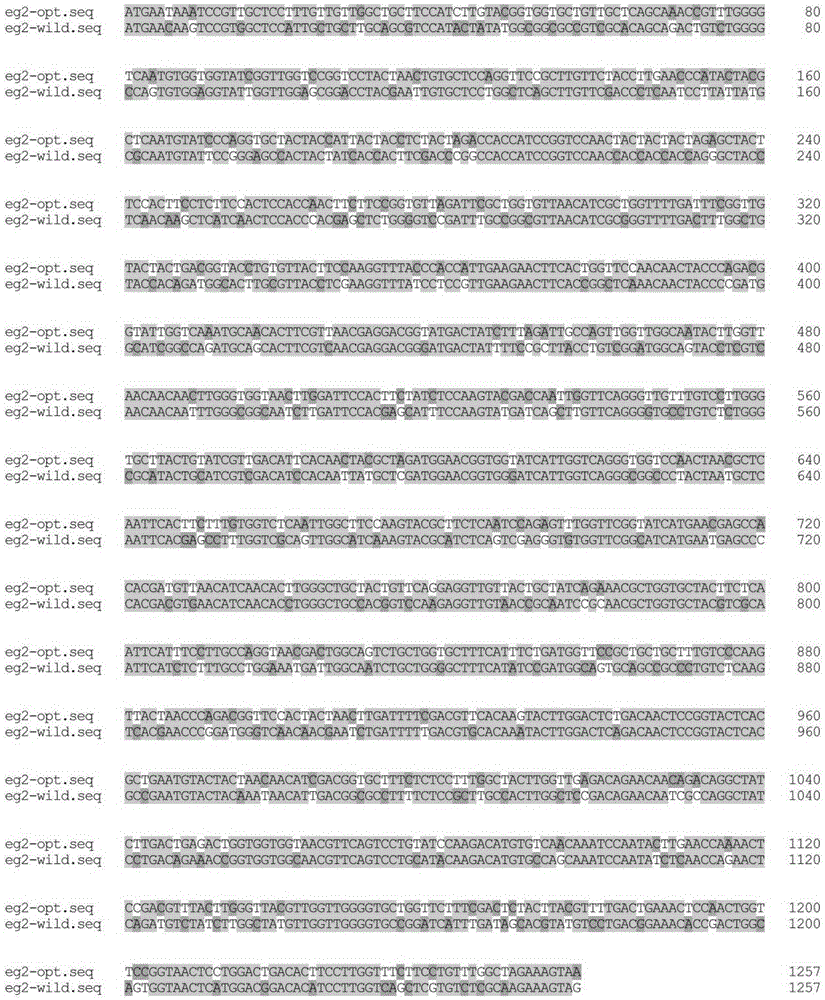
[0063] 1, the present invention utilizes GeneDesigner (DNA2.0, MenloPark, CA, USA) to realize as shown in SEQIDNO.1 EGII (GenBankAccessionNo.DQ178347.1) nucleotide sequence codon bias optimization, optimized nucleotide sequence such as SEQIDNO .3 shown. The codon-optimized amino acid sequence is consistent with the original amino acid sequence, as shown in SEQ ID NO.2. Using Pichiapastoris GS115 as the host, the nucleotide sequence after codon bias optimization and the original nucleotide sequence are compared, see figure 2 , compare the amino acid sequence after codon bias optimization with the original amino acid sequence, see image 3 .
[0064] 2. With EcoRI as the 5'-end restriction site and NotI as the double-restriction site as the 3'-end restriction site, insert the pPIC9K plasmid vector downstream of the AOX1 promoter to construct the expressi...
Embodiment 2
[0065] Example 2: Linearizing expression vectors and performing electrotransformation and screening high-yielding strains.
[0066] 1. Use SacI as the linearization site of the pPIC9K-eg2 expression vector to realize the linearization of the expression vector, and verify the linearized plasmid by nucleic acid electrophoresis, see Figure 5 . Transform into Pichiapastoris GS115 by electroporation to obtain recombinant strains, use geneticin G-418 concentration gradient to screen multi-copy transformants, and obtain high-yield strains by shake-flask fermentation of colonies that can grow normally on high-concentration resistance plates. Determination of CMC enzyme activity in fermentation supernatant by DNS method, see Figure 6 , wherein, 1 unit of enzyme activity is defined as the amount of enzyme that can convert 1 μmol of substrate in 1 minute under the condition of 50°C water bath.
Embodiment 3
[0067] Example 3: Acquisition and expression vector construction of Vitella hyaline hemoglobin (VHb) gene.
[0068] 1. Obtain the Vitiligo hyaline hemoglobin gene (GenBankAccessionNo.M30794.1) from NCBI, add an EcoRI restriction site at the 5' end, add a NotI restriction site at the 3' end, and obtain the target gene by artificial synthesis.
[0069] 2. With EcoRI as the 5'-end restriction site and NotI as the 3'-end restriction site as the double restriction site, insert the pPICZαA plasmid vector downstream of the AOX1 promoter to construct the expression vector pPICZαA-vgb. See the expression vector map Figure 7 . The expression vector was verified by double enzyme digestion to confirm that the expression vector pPICZαA-vgb was successfully constructed. See the nucleic acid electrophoresis diagram after enzyme digestion Figure 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com