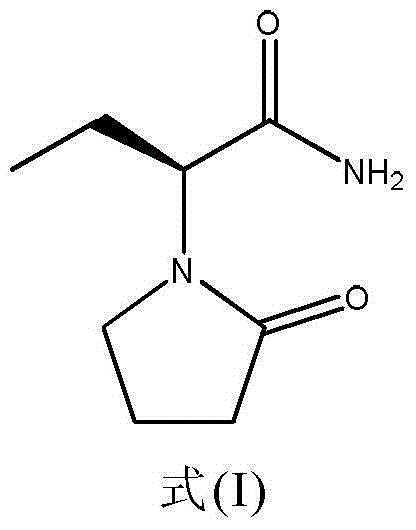
Levetiracetam tablet and preparation method thereof
A tablet and filler technology, applied in the field of pharmaceutical preparations, can solve the problems of complex process, poor fluidity, drug failure, etc., and achieve the effects of stable preparation process, reduced production cost, and strong industrial feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
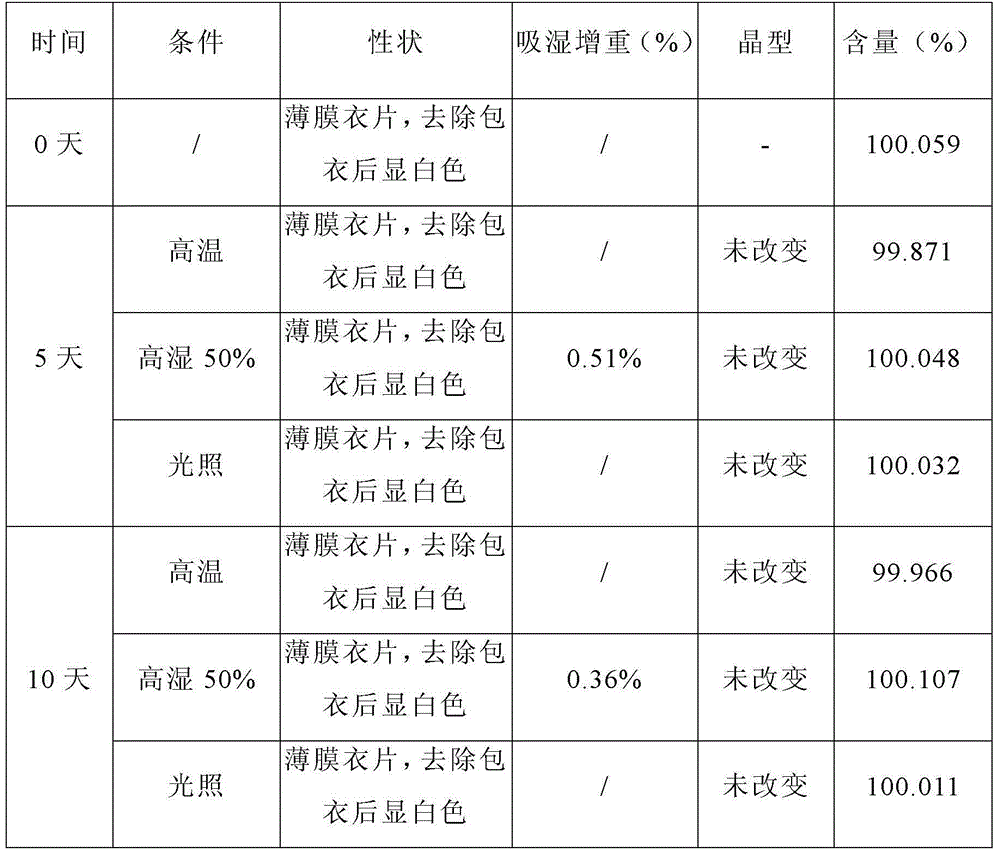
[0049] Embodiment 1: levetiracetam tablet
[0050] prescription:
[0051]
[0052] Preparation:
[0053] (1) The raw and auxiliary materials are respectively pulverized and passed through a 100-mesh sieve for subsequent use;
[0054] (2) take povidone K30, be mixed with 10% (weight) aqueous solution;
[0055] (3) Accurately weigh each raw and auxiliary material according to the prescription amount, mix levetiracetam with lactose, starch, and sodium carboxymethyl starch, add an appropriate amount of 10% povidone K30 aqueous solution, and make a soft material;
[0056] (4) Soft materials are passed through a 20-mesh sieve to prepare wet granules, and dried at 60°C until the moisture content of the granules is 2-4%;
[0057] (5) The dry granules are granulated through a 20-mesh sieve, and the prescription amount of magnesium stearate and silicon dioxide are added, and mixed uniformly;
[0058] (6) Determination of main drug content, calculation of tablet weight;
[0059] ...
Embodiment 2
[0060] Embodiment 2: Levetiracetam film-coated tablet
[0061] Coating Solution Prescription:
[0062]
[0063] Coating process:
[0064] (1) Coating liquid preparation: add the calculated purified water into the liquid preparation container, start the agitator, the height of the stirring blade from the bottom of the container is 1 / 3 of the liquid height, so that the liquid is completely stirred, and the liquid surface just forms a vortex Without splashing the liquid, sprinkle the coating powder on the vortex liquid surface at a steady speed, keep stirring for 45 minutes until the coating agent is completely dissolved, pass through a 100-mesh sieve, and set aside;
[0065] (2) Coating: Add the tablet core obtained in Example 1 to the coating pot, adjust the pot speed, start the hot air system, preheat, open the spray gun and spray the prepared coating powder solution, check the coating of the plain tablet at any time. To the degree of coating, control the weight gain of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com