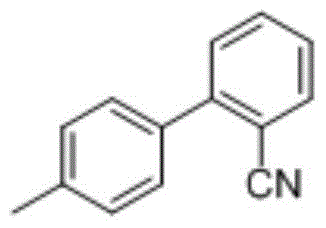
The preparation method of 2-cyano-4'-methylbiphenyl
A technology of methyl biphenyl and p-toluene boronic acid, which is applied in the field of preparation of 2-cyano-4'-methyl biphenyl, can solve the problems of complex catalyst preparation, poor recyclability, and high cost of use, and achieve reaction Mild conditions, high-efficiency catalysis, and environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, the preparation method of polystyrene-triphenylphosphine:
[0021] Add 2.0 g (6.9 mmol) of diphenyl (4-vinylphenyl) phosphine, 2.9 ml (27.6 mmol) of styrene, and 3 mL of toluene to a 10 mL three-necked flask, dissolve them, and add 78.0 mg of AIBN (azobisisobutyl Nitrile), N 2 Under protection, stirred and heated to 70°C for 24 hours, TLC detected the disappearance of diphenyl (4-vinylphenyl) phosphine in the reaction solution; concentrated to remove the solvent, dissolved the obtained solid in 6 mL THF (tetrahydrofuran), and added dropwise (about 5 minutes) Added) into 60ml of methanol at 0°C, a white solid was precipitated, filtered with suction to obtain 4.73g of polystyrene-triphenylphosphine compound (III) in the form of white powder, yield: 97%.
Embodiment 2
[0022] Embodiment 2, the preparation method of polystyrene-triphenylphosphine supported palladium catalyst:
[0023] Add 1g of compound (III) and 10ml of THF to a 25ml reaction flask, heat up to 50°C, and then add 84.0mg (0.375mmol) of Pd(OAc) 2 , continue to react at 50°C for 4h, cool to 0°C, add this solution dropwise (about 10 minutes to complete) into 20ml of hexane at 0°C, stir at room temperature for 12h, filter with suction, filter the cake with 5ml of methanol After washing, 1.05 g of black solid polystyrene-triphenylphosphine supported palladium compound was obtained, yield: 97%.
Embodiment 3
[0024] The preparation method of embodiment 3,2-cyano-4'-methylbiphenyl:
[0025] 1.376g (10mmol) of o-chlorobenzonitrile, 1.700g (12.5mmol) of p-tolueneboronic acid and 2.764g (20mmol) of anhydrous sodium carbonate were added to a container containing 100mL EtOH / H 2 In a three-neck flask of O (v / v, 1 / 1), heat to reflux, add 41.4mg of polystyrene-triphenylphosphine supported palladium catalyst, continue to heat and reflux for 1h, TLC detects that the reaction liquid o-chlorobenzonitrile disappears, and suction filter , the filter cake was washed with 3×20ml ethyl acetate, 2×4ml EtOH / H 2 O (v / v, 1 / 1) washing, drying at room temperature to constant weight, reclaiming polystyrene-triphenylphosphine supported palladium catalyst 40.2mg, recovery rate: 97%; the filtrate gained by suction filtration was extracted with 40ml water , the organic phase (at the upper layer) was washed with 20 ml of saturated brine, and the washed organic phase was dried with 2.0 g of anhydrous sodium sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com