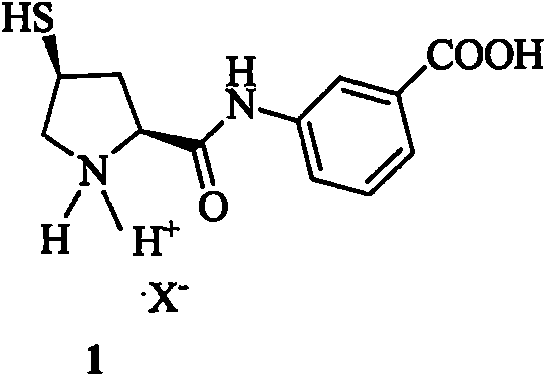
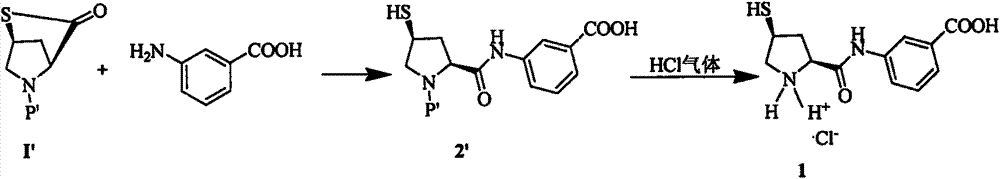
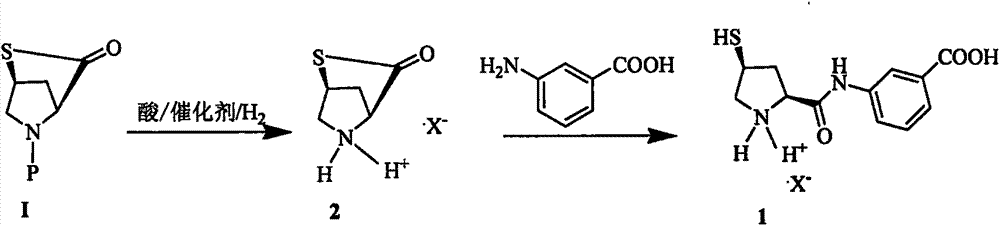
Novel preparation method of ertapenem side chain
A kind of ertapenem side chain, new technology, applied in the field of preparation of carbapenem antibiotic side chain, can solve the problems such as not easy to be used in mass production, low yield of raw material I′ preparation process, not easy to obtain, etc., to achieve easy industrialization The effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of compound 2
[0028] In the 500mL hydrogenation kettle, add methanol 200g, raw material I15.4g (0.05mol), 4M HCl methanol solution 30ml (0.12mol), 10%Pt / SiO 2 7.6g. Nitrogen was replaced several times, hydrogen was replaced several times, and finally hydrogen was passed to the pressure of 1.5 MPa in the kettle, the temperature was controlled at 40°C, and the reaction was stirred for 1 h. Stirring was stopped, hydrogen was vented and replaced with nitrogen. Filtrate, recover and reuse the filter cake; slowly add 400ml of methylcyclohexane dropwise to the filtrate at 40°C, and after solid precipitation, slowly cool down to 10°C for crystallization for 2h, filter, and vacuum-dry to obtain 27.50g of off-white solid compound , 90% molar yield.
[0029] 1 H-NMR (400MHz, D 2 O): δ2.12(m, 1H), 2.94(m, 1H), 3.30(m, 1H), 3.64(m, 1H), 3.72(m, 1H), 4.57(t, J=8.4Hz, 1H ), 4.65 (brs, 1H)
Embodiment 2
[0030] Embodiment 2: the preparation of compound 2
[0031] Add 300 g of ethanol, 18.5 g (0.06 mol) of raw material I, 37.5 mL (0.15 mol) of 4M / HCl ethanol solution, 10% Pt / γ-Al in the 500 mL hydrogenation kettle 2 o 3 5.0 g. Nitrogen was replaced several times, hydrogen was replaced several times, and finally hydrogen was passed to the pressure of 1.2MPa in the kettle, the temperature was controlled at 50°C, and the reaction was stirred for 1.5h. Stirring was stopped, hydrogen was vented and replaced with nitrogen. Filter and recover the filter cake for reuse; slowly add 600 mL of n-hexane dropwise to the filtrate at 30 °C, stir for 30 min, and after solid precipitation, slowly cool down to 0 °C for crystallization for 1.5 h, filter, and vacuum dry to obtain off-white solid compound 29.32 g, molar yield 92%
Embodiment 3
[0032] Embodiment 3: the preparation of compound 2
[0033] Add THF150g and purified water 100g, raw material I12.32g (0.04mol), concentrated hydrochloric acid 10mL (0.12mol), 10%Pt / γ-Al in 500mL hydrogenation kettle 2 o 3 6g. Nitrogen was replaced several times, hydrogen was replaced several times, and finally hydrogen was passed to the pressure in the kettle to 2 MPa, the temperature was controlled at 30°C, and the reaction was stirred for 2 hours. Stirring was stopped, hydrogen was vented and replaced with nitrogen. Filtration, filter cake recycling; filtrate with 5% NaHCO 3 Adjust the pH of the liquid to 8-9, let stand to separate the liquids, wash the upper layer with purified water, dry over anhydrous sodium sulfide, filter, adjust the filtrate to 3-4 with 4M HCl methanol solution, concentrate, add 50 mL of ethyl acetate and normal Heptane 100mL was stirred and crystallized at 20°C for 2h to obtain 25.72g of off-white solid compound with a molar yield of 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com