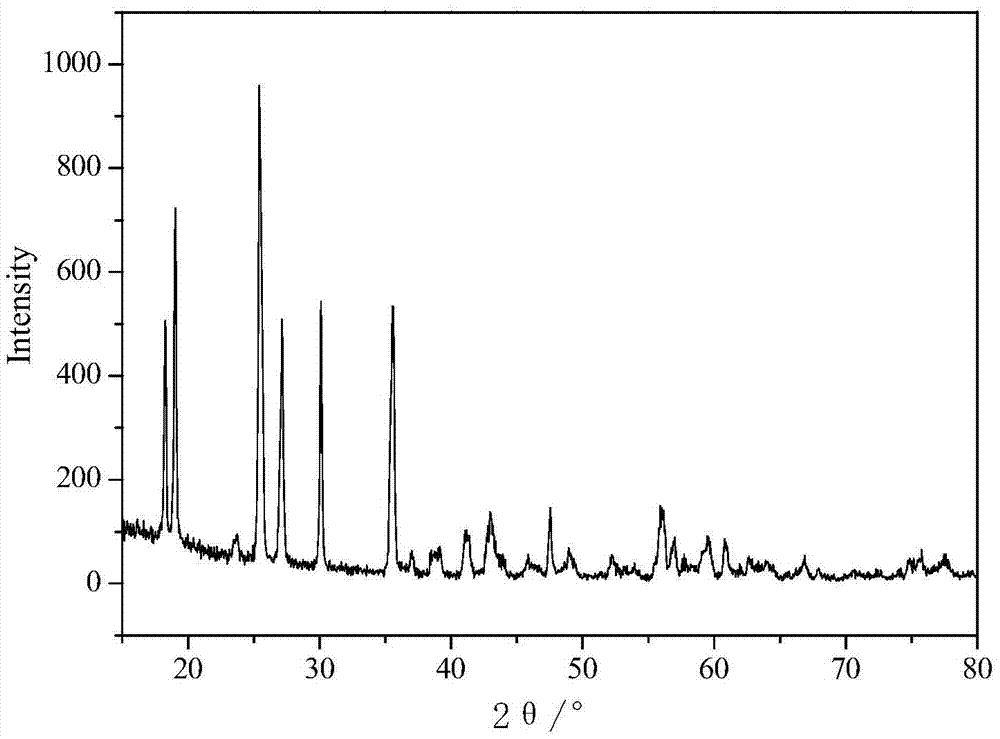
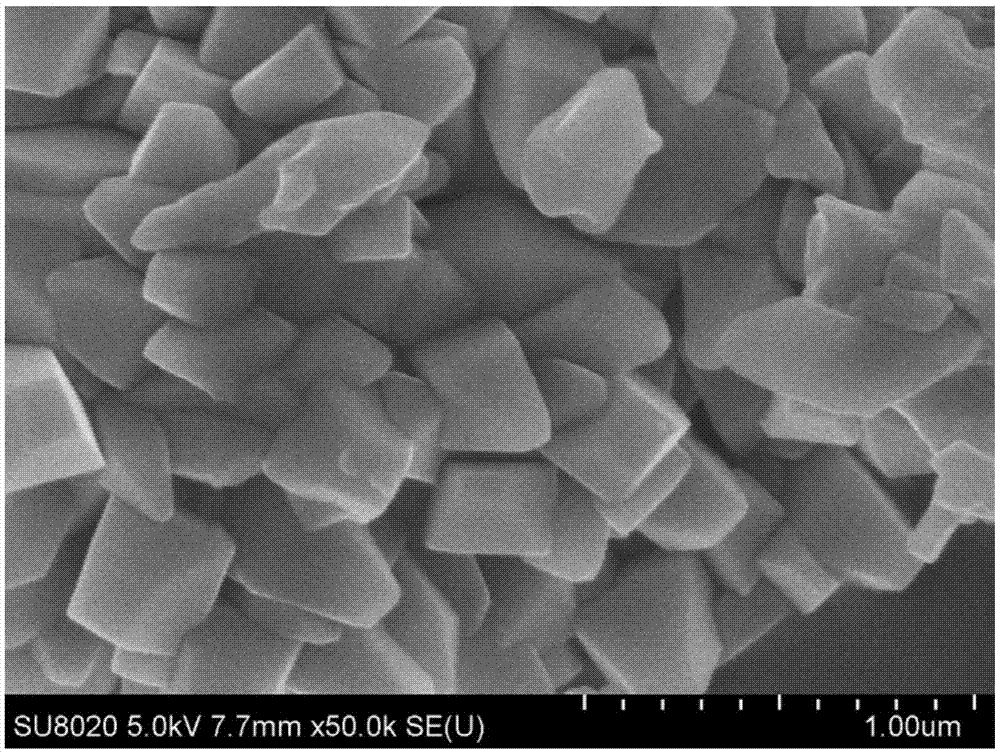
Preparation method of manganese iron phosphate and product
A technology of iron manganese phosphate and iron source, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of high reaction height, high equipment requirements, wide particle size distribution, etc., and achieve uniform particle size distribution and high reaction temperature Low, high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 100.00g of manganese sulfate and 48.03g of ferric chloride and dissolve them in 300ml of pure water respectively, and add 60.91ml and 30.46ml of phosphoric acid respectively, then transfer the solution to the reaction kettle and adjust its pH=1.5; then add Oxidant hydrogen peroxide 83.83ml and dispersant ethanol 10ml, temperature rises to 85 ℃, reacts for 24 hours; After the reaction, the obtained ferromanganese phosphate slurry is washed with water, filtered and dried to obtain the final ferromanganese phosphate (with or without crystal water )product.
Embodiment 2
[0028] Weigh 100.00g of manganese sulfate and 37.53g of ferrous chloride and dissolve them in 300ml of pure water respectively, and add 60.91ml and 30.46ml of phosphoric acid respectively, then transfer the solution to the reactor and adjust its pH=1.0; then Add 125.74ml of oxidant hydrogen peroxide and 10ml of dispersant ethanol, raise the temperature to 90°C, and react for 16 hours; after the reaction, the obtained ferromanganese phosphate slurry is washed with water, filtered, and dried to obtain the final ferromanganese phosphate (with or without crystallization). water) products.
Embodiment 3
[0030] Weigh 100.00g of manganese sulfate and 41.10g of ferrous sulfate and dissolve them in 300ml of pure water respectively, and add 60.91ml and 15.22ml of phosphoric acid respectively, then transfer the solution to the reactor and adjust its pH=1.5; then add Oxidant hydrogen peroxide 104.79ml and dispersant ethanol 15ml, the temperature is raised to 105 DEG C, react for 10 hours; After the reaction, the obtained ferromanganese phosphate slurry is washed with water, filtered and dried to obtain the final ferromanganese phosphate (with or without crystal water )product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com