Enzalutamide preparation method
A technology of enzalutamide and triethylamine, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of non-production process, high cost, and low reaction yield, and achieve the effects of reducing production cost, reducing the generation of impurities, and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
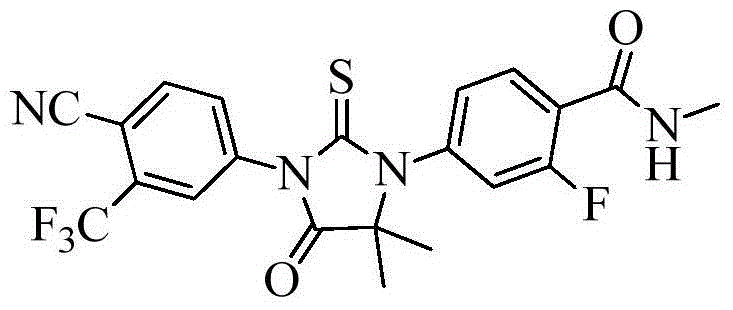
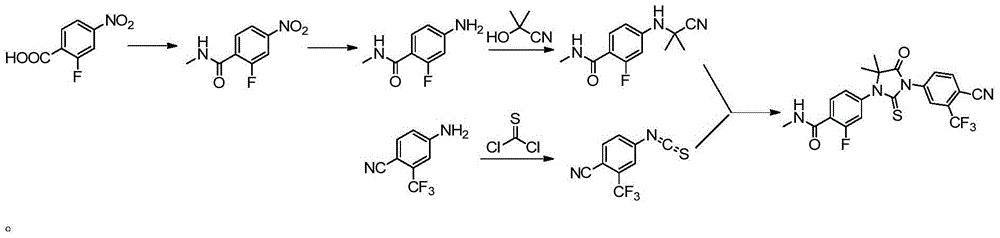
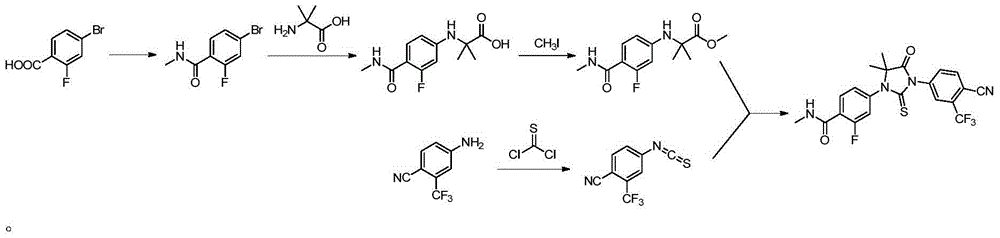
[0031] Example 1: 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioimidazolidin-1-yl )-2-fluoro-N-methylbenzamide preparation
[0032] 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropionic acid (5.08g, 0.02mol, 1eq), triethylamine (2.42g, 0.024mol, 1.2eq), 4-isothiocyanoyl-2-(trifluoromethyl)benzonitrile (5.47g, 0.024mol, 1.2eq) and chloroform (100mL) were added in a 250mL reaction flask, and the temperature was raised to 50 The reaction was carried out under reflux at ℃ for 2 hours, and the progress of the reaction was monitored by TLC (n-hexane:ethyl acetate=1:1). After the reaction was completed, cool to room temperature, wash the chloroform layer successively with 1mol / L sodium hydroxide solution (once), and saturated brine (twice), dry over anhydrous sodium sulfate, filter, and concentrate to obtain a yellow-white solid. Add 50ml of isopropanol to the distillate, raise the temperature to reflux and stir to dissolve, cool to room temperature to...
Embodiment 2
[0035] Example 2: 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioimidazolidin-1-yl )-2-fluoro-N-methylbenzamide preparation
[0036] 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropionic acid (2.54g, 0.01mol, 1eq), triethylamine (1.21g, 0.012mol, 1.2eq), 4-isothiocyanato-2-(trifluoromethyl)benzonitrile (2.73g, 0.012mol, 1.2eq) and dichloromethane (50mL) were added in a 100mL reaction flask, and the temperature was raised to 40 The reaction was carried out under reflux at ℃ for 5 hours, and the progress of the reaction was monitored by TLC (n-hexane:ethyl acetate=1:1). After the reaction was completed, cool to room temperature, wash the dichloromethane layer successively with 1mol / L sodium hydroxide solution (once), and saturated brine (twice), dry over anhydrous sodium sulfate, filter, and concentrate to obtain a yellow-white solid, which was sent to Add 25ml of isopropanol to the fraction, raise the temperature to reflux and stir to dissolve, co...
Embodiment 3
[0037] Example 3: 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioimidazolidin-1-yl )-2-fluoro-N-methylbenzamide preparation
[0038] 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropionic acid (2.5g, 0.01mol, 1eq), triethylamine (1.2g, 0.012mol, 1.2eq), 4-isothiocyanato-2-(trifluoromethyl)benzonitrile (2.7g, 0.012mol, 1.2eq) and tetrahydrofuran (50mL) were added to a 100mL reaction flask, heated to 60°C and refluxed The reaction was carried out for 3 hours, and the progress of the reaction was monitored by TLC (n-hexane:ethyl acetate=1:1). After completion of the reaction, concentrate under reduced pressure to dryness, cool to room temperature, add 100 ml of dichloromethane to the fraction, wash the dichloromethane layer with 1mol / L sodium hydroxide solution (once) and saturated brine (twice) successively, Dry over anhydrous sodium sulfate, filter, and concentrate to obtain a reddish-yellow oil. Add 25ml of isopropanol to the fraction, heat up to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com