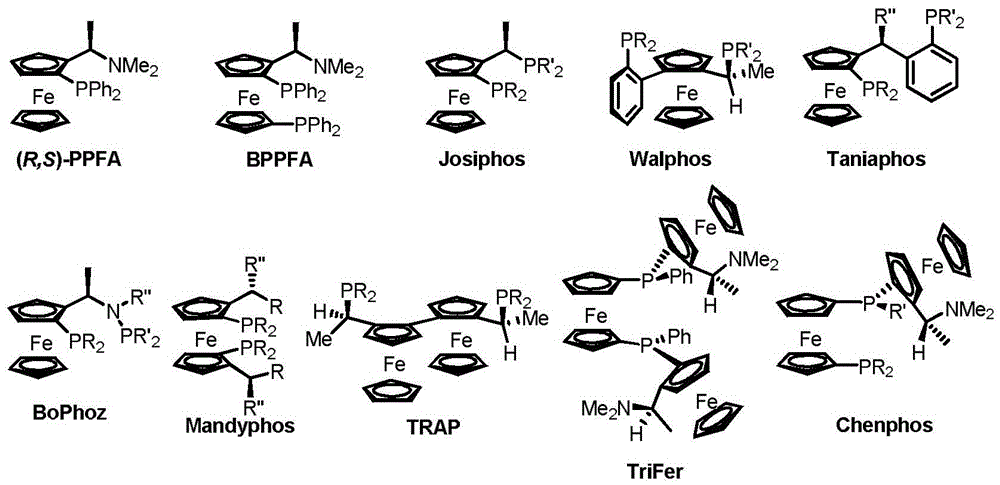
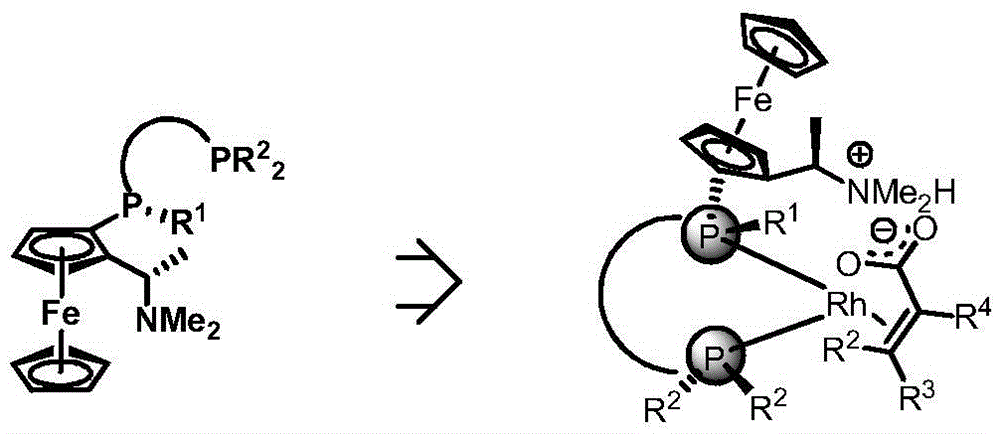
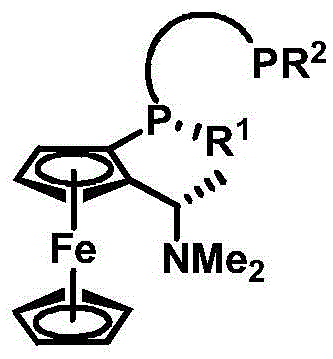
Chiral diphosphine ligand and application thereof to asymmetric hydrogenation and correlated reactions
A technology of chiral bisphosphine ligands and chiral phosphine ligands, applied in the field of chiral bidentate phosphine ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 intermediate 1:
[0034]
[0035] (s)-2-Dimethylamino-ethylferrocene (2.5715 g) and 10 mL of anhydrous diethyl ether were sequentially added to a 100 mL Schlunk flask replaced with a nitrogen atmosphere. To the obtained solution was added dropwise 1.5M tert-butyl lithium in pentane solution (7.6 mL) at -78°C, and the reaction was carried out at room temperature for 1.5 h. After the reaction liquid was cooled to -78°C, phosphine trichloride (1 mL) was added, and the resulting reaction liquid was raised to room temperature for 1.5 h. After the reaction solution was cooled to -78°C, 2-bromo-phenylmagnesium chloride (10 mmol) prepared in advance was added, and the reaction was carried out at room temperature for 1.5 h. After cooling the reaction solution to -78°C again, 3.0M methylmagnesium chloride (3.8mL) was added, and the resulting solution was raised to room temperature and reacted for 3h, then 10mL of water was added to separate the...
Embodiment 2
[0036] The preparation of embodiment 2 ligand L1:
[0037]
[0038] Intermediate 1 (1.3746 g) and 20 mL of anhydrous diethyl ether were sequentially added into a 50 mL Schlunk flask replaced with a nitrogen atmosphere. To the obtained solution was added dropwise 2.3M n-butyllithium hexane solution (1.4 mL) at -78°C, and the mixture was raised to room temperature for 1 h. After the reaction solution was cooled to -78°C, diphenylphosphine chloride (704 mg) was added, and the resulting reaction solution was raised to room temperature for 3 hours, then 20 mL of water was added, the organic phase was separated, and the aqueous phase was extracted three times with ethyl acetate (each 20mL). The obtained organic phase was dried over anhydrous sodium sulfate and then spin-dried under reduced pressure. The crude product was purified by column chromatography to obtain the target product ligand L1 (1.40 g, yield 83%).
Embodiment 3
[0039] The preparation of embodiment 3 ligand L2:
[0040]
[0041] Intermediate 1 (1.3746 g) and 20 mL of anhydrous diethyl ether were sequentially added into a 50 mL Schlunk flask replaced with a nitrogen atmosphere. To the obtained solution was added dropwise 2.3M n-butyllithium hexane solution (1.4 mL) at -78°C, and the mixture was raised to room temperature for 1 h. After cooling the reaction solution to -78°C, dicyclohexylphosphine chloride (767.9 mg) was added, and the resulting reaction solution rose to room temperature and reacted for 3 hours, then 20 mL of water was added to separate the organic phase, and the aqueous phase was extracted three times with ethyl acetate (each times 20mL). The obtained organic phase was dried over anhydrous sodium sulfate and then spin-dried under reduced pressure. The crude product was purified by column chromatography to obtain the target product ligand L2 (1.47 g, yield 85%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com