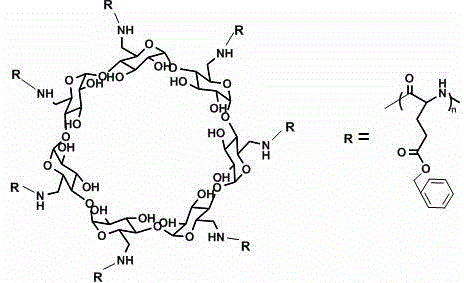
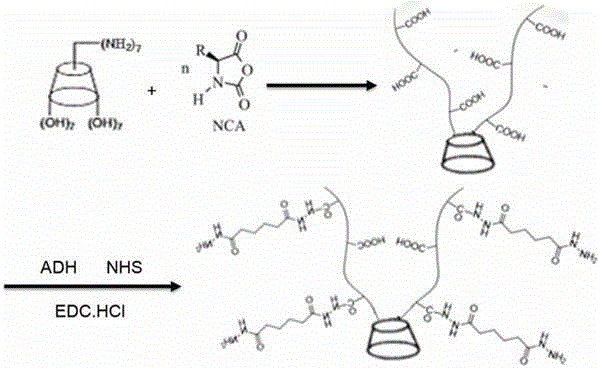
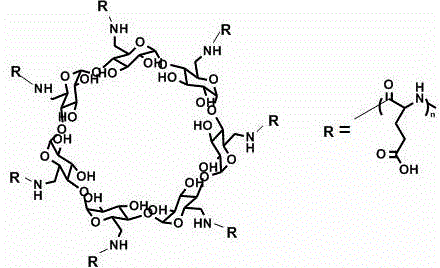
Star-shaped polyglutamic acid as well as injectable hydrogel and preparation method thereof
A technology of polyglutamic acid and star structure, applied in the field of polyglutamic acid with star structure, injectable hydrogel and its preparation, can solve the problem of poor compatibility, affecting mechanical properties and dimensional stability, release Dose and dynamics are difficult to control and other issues, to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Take a dry 250ml round bottom flask, add 40ml DMF and 10.2g triphenylphosphine, after fully dissolved, slowly add 10.2g iodine under ice bath condition, the system turns dark brown, then the temperature gradually rises to 50°C, add 2.9gβ- Cyclodextrin, stirred and reacted for 18 hours under an inert gas atmosphere. Then stop heating, distill 20ml of DMF under reduced pressure, and then ice bath, add 10ml of sodium methylate to the flask to terminate the reaction, add 200ml of anhydrous methanol to settle the reaction after 30 minutes, filter, wash with methanol for several times and then vacuum dry to obtain a white powder Shaped solid 3.5g, that is, polyiodide β-cyclodextrin.
[0032] Dissolve 2.9g of polyiodo-β-cyclodextrin in 50ml of DMF, add 1g of sodium azide, stir at 60°C for 20 hours under inert gas conditions, then distill 25ml of DMF under reduced pressure, pour into 500ml of water to settle, filter, After washing several times and drying in vacuum, a white po...
Embodiment 2
[0039] This example is basically the same as Example 1, except that in Step 4, 0.26g of polyamino β-cyclodextrin is added, 50ml of dioxane treated with calcium hydride to remove water is injected, and the suspension is obtained by stirring , Add 5 g of L-glutamic acid-N-carboxylic acid anhydride. The finally obtained polyglutamic acid with a star structure has a molecular weight of about 23000 Daltons. The dosage and operating conditions of other reagents remained unchanged. The finally obtained injectable hydrogel had a gelation time of 242s and an elastic modulus of 0.72KPa.
Embodiment 3
[0041] This example is basically the same as Example 1, except that in Step 4, 2.3g of polyamino β-cyclodextrin was added, 50ml of dioxane treated with calcium hydride to remove water was injected, and the suspension was obtained by stirring , Add 5 g of L-glutamic acid-N-carboxylic acid anhydride. The finally obtained polyglutamic acid with a star structure has a molecular weight of about 14,000 Daltons. The dosage and operating conditions of other reagents remained unchanged. The finally obtained injectable hydrogel had a gelation time of 260s and an elastic modulus of 0.7KPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com