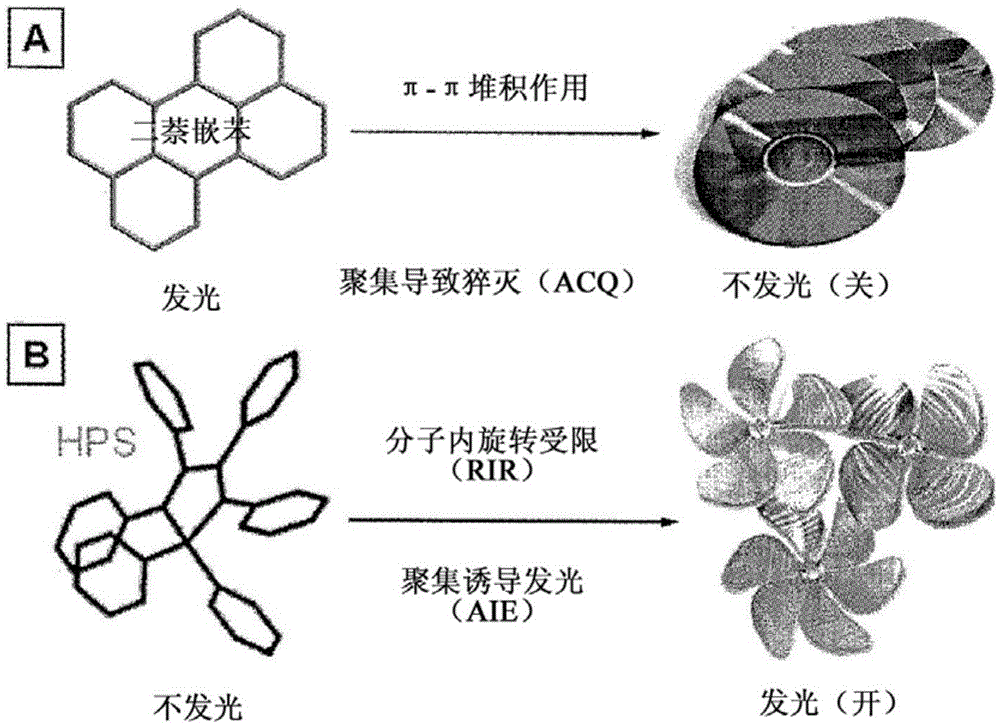
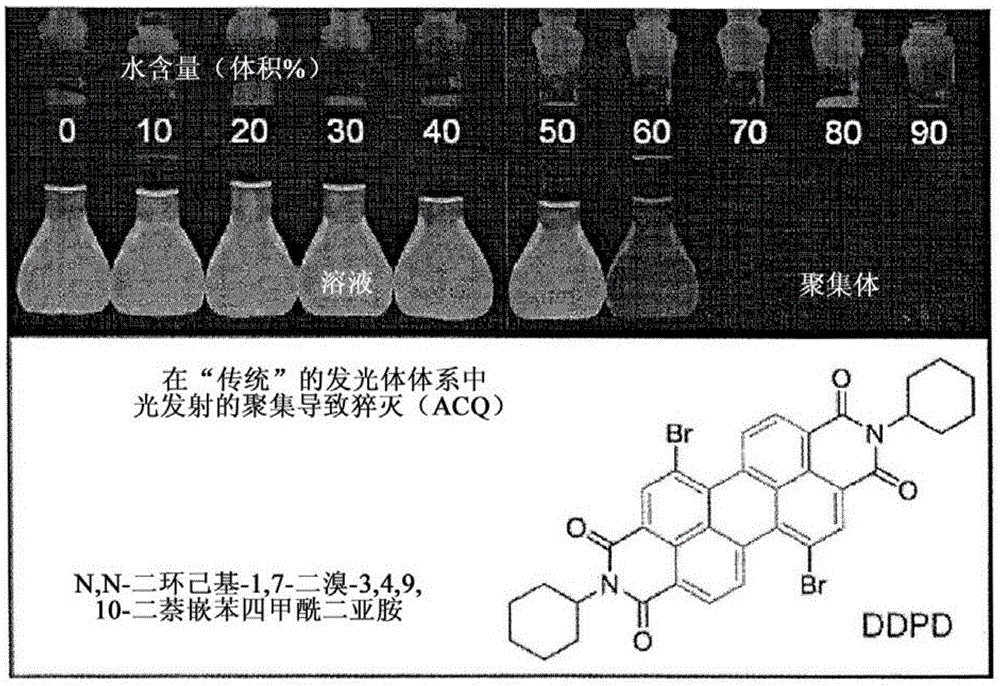
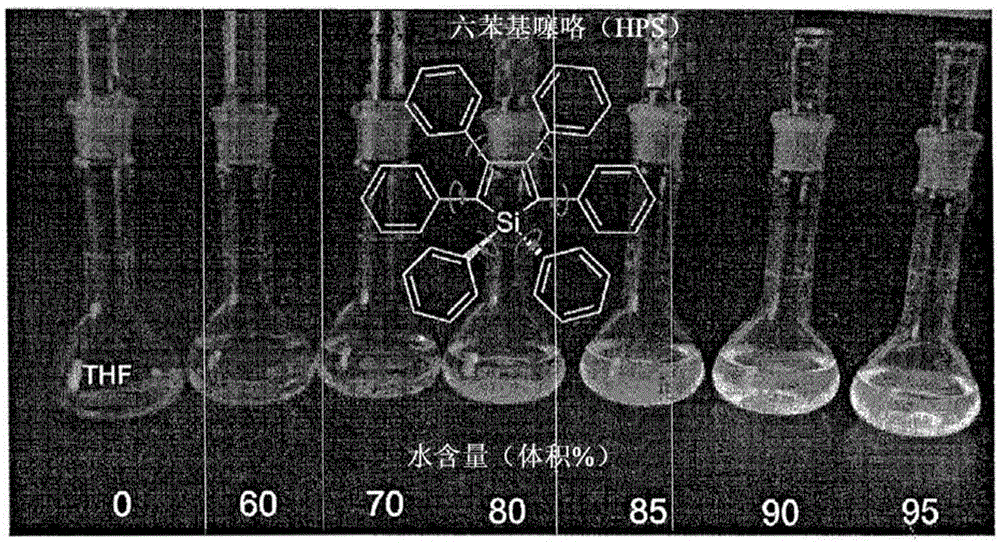
Composition and synthesis of aggregation-induced emission materials
A technology of synthesis and luminescence, which is applied in the direction of luminescent materials, compounds of group 5/15 elements of the periodic table, analytical materials, etc., and can solve the problem of confinement effects, fluorescence quenching, and difficult detection of low-abundance molecular species in biological systems, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0206] In addition, the subject matter of the present application also discloses a method for synthesizing TPE derivatives with activated luminophores showing aggregation-induced luminescence, comprising the steps of: (a) adding benzoyl chloride to DBT or DBF; (b) refluxing the reaction mixture to obtain an intermediate compound; (c) subjecting the intermediate compound to a McMurry coupling reaction to obtain a TPE derivative. In some embodiments, the intermediate compound is dibenzo[b,d]thiophen-2-yl(phenyl)methanone or dibenzo[b,d]furan-2-yl(phenyl)methanone. In another embodiment, aluminum chloride or dichloromethane is added to step (a) of the synthesis process of the TPE derivative.
[0207] In one embodiment, when DBT is used in step (a), the E-isomer of STPE is formed in DCM and methanol solution, wherein in step (a) DBT is dissolved in DCM solution, methanol is gradually Dropwise into this solution. In another embodiment, when DBT is used in step (a), the Z-isomer i...
Embodiment
[0299] Preparation of TPE and its derivatives
[0300] Benzophenone, palladium activated carbon (Pd / C), zinc powder, dibenzosubrone and all benzophenone derivatives were purchased from Aldrich and used without further purification. Tetrahydrofuran (THF) was freshly distilled from sodium benzophenoneketyl under nitrogen atmosphere and used immediately.
[0301] Measured on a Bruker AV300 spectrometer using tetramethylsilane (TMS; δ=0) as internal standard in deuterated chloroform 1 H and13 CNMR spectrum. Absorption spectra were measured on a MiltonRoy Spectronic3000 array spectrophotometer. Photoluminescence was recorded on a Perkin-Elmer LS55 spectrofluorometer. High resolution mass spectra (HRMS) were recorded on a GCTpremierCAB048 mass spectrometer operated in MALDI-TOF mode. Single crystal X-ray diffraction intensity data were collected at 100K on a Bruker-Nonices SmartApex CCD diffractometer with graphite monochromatized MoKα radiation. The intensity data were proce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com