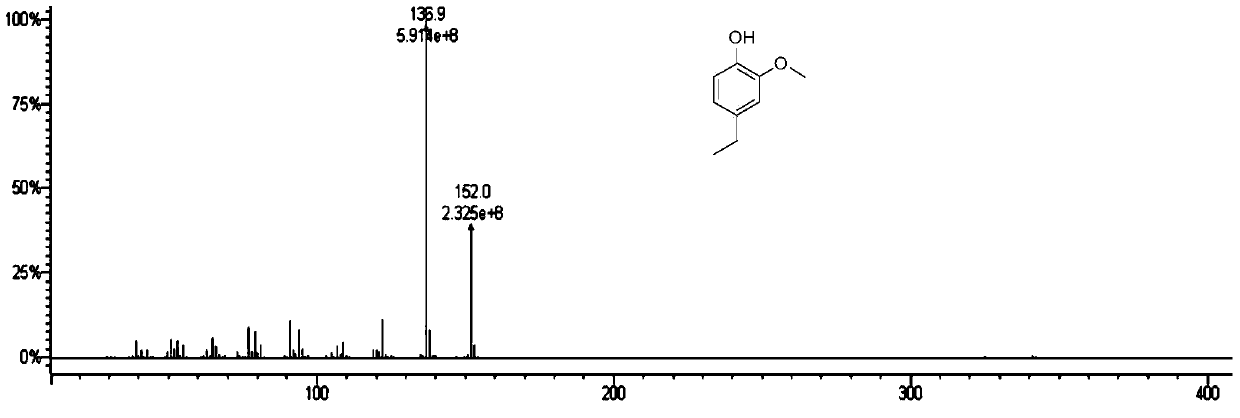
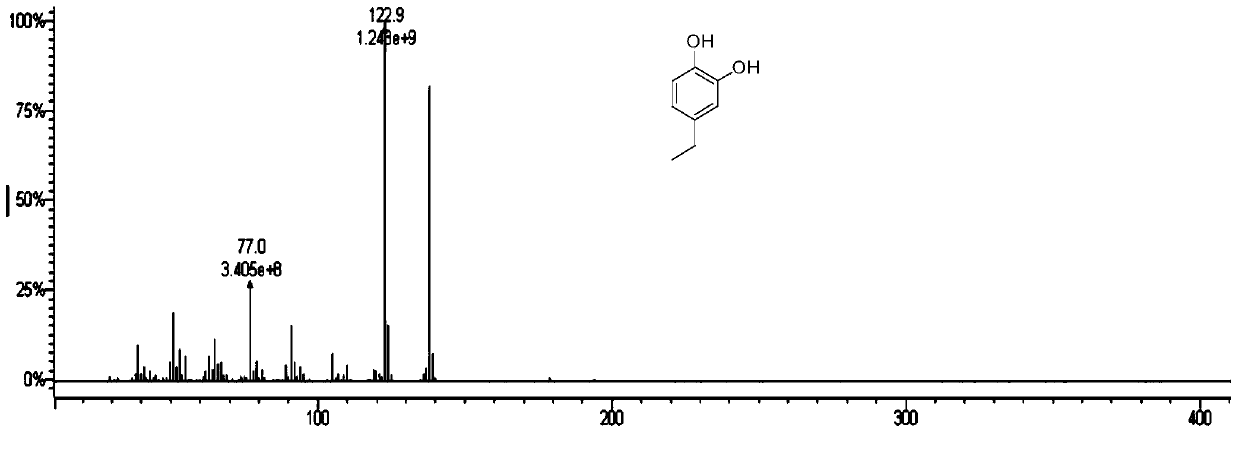
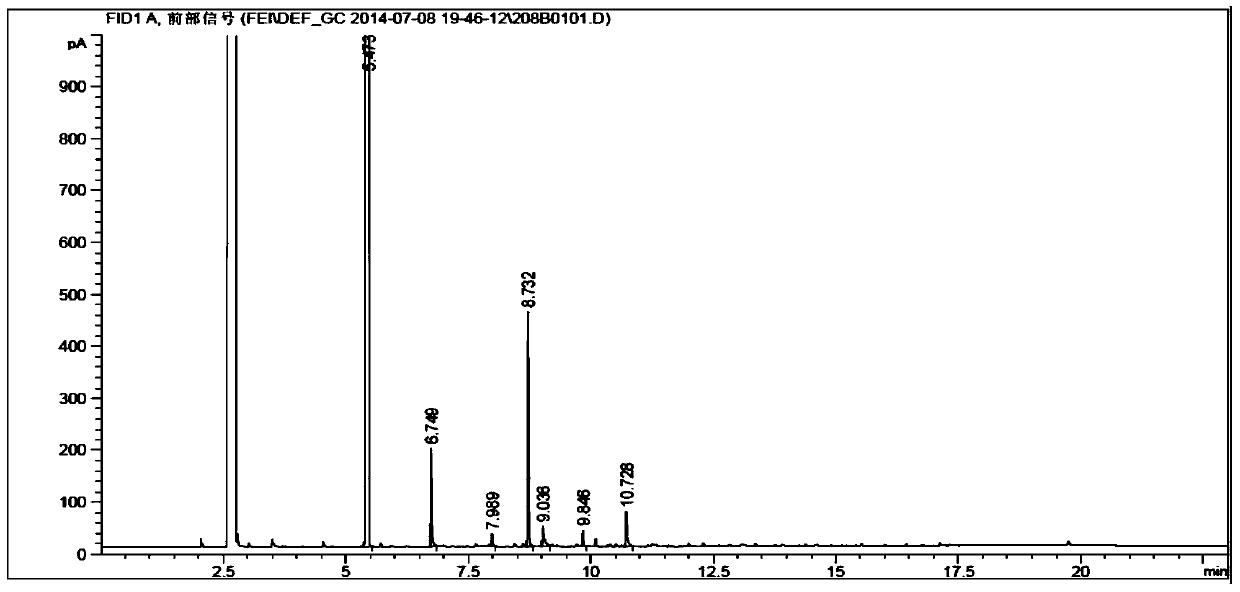
Method for preparation of benzene ring phenol compound from alkali lignin
A technology for alkali lignin and phenol, which is applied in the field of preparing phenol compounds, can solve the problems of reducing reaction activity and selectivity, improving reaction risk coefficient and the like, and achieves the effects of simple recovery, cheap raw materials, and easy separation of products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Metal catalyst: commercial catalysts can be used, such as: the catalyst Raney nickel used in the examples is commercially purchased from Anshan Zhongli Catalyst Factory, model ZL-N311, Ni content ≥ 90%.
[0041] The impregnation method can also be used to take metal precursors of different concentrations according to different loadings, and make a self-made supported metal catalyst. Taking the preparation of Pd / C and Ru / C as an example: in the corresponding metal precursors Palladium chloride and The aqueous solution of ruthenium trichloride impregnates the active carbon carrier in a medium volume, stirs it evenly, and then dries it at 60°C, then dries it at 120°C for 12 hours, and finally reduces it in a hydrogen atmosphere at 450°C for 1 hour, and then uses O 2 1% O 2 / N 2 The metal catalyst can be obtained by passivating the mixed gas for 6 hours.
Embodiment 2-10
[0043] Weigh a total of 10 grams of phosphorylcholine or a mixed solvent and transfer it to a 75 milliliter reaction kettle, add 0.25 grams of alkali lignin and 0.05 grams of Raney nickel catalyst, replace it with hydrogen for 3 times, and fill it with a certain amount of pressure of hydrogen , the reaction temperature is raised to a certain temperature, stirred quickly at 800rpm, and the heating is stopped after 6 hours of reaction. When the temperature drops to room temperature, the vent valve is opened to reduce the pressure in the kettle to normal pressure. Extract 3 times, use gas chromatography to analyze product distribution, and remove ethyl acetate with a rotary evaporator, and weigh it as the total weight of the product.
[0044] Conversion rate X (%) calculation method:
[0045] The reaction conditions and reaction results are shown in Table 1.
[0046] Table 1
[0047]
[0048] As shown in Table 1, the reaction medium used in the reaction temperature range o...
Embodiment 11-16
[0050] Adopt the reaction condition of embodiment 2-10, only change catalyst, and reaction condition and reaction result are shown in Table 2 for details.
[0051] Table 2
[0052]
[0053] As shown in Table 2, alkali lignin can be converted to ethylphenol with high selectivity on different catalysts in the catalytic process involved in the present invention. Among them, the selectivity of ethylphenol on the Raney nickel catalyst and the Pd catalyst can reach 47% and 48%, which shows that it is better than other catalysts in the phosphorylcholine composite medium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com