The preparation method of trifluoromethylbenzothiophene derivative
A technology for thiophene derivatives and trifluoromethylbenzene is applied in the field of preparation of trifluoromethyl-containing benzothiophene derivatives, which can solve the problems of pollution in the preparation process, complicated technological process and high production cost, and achieve a cheap synthesis system. , the process is simple, the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
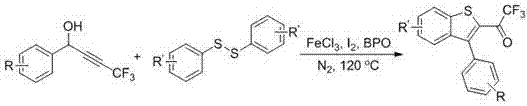
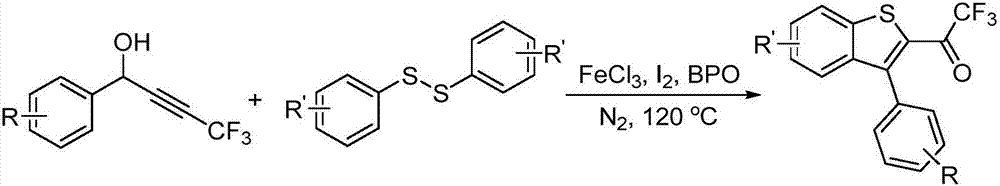
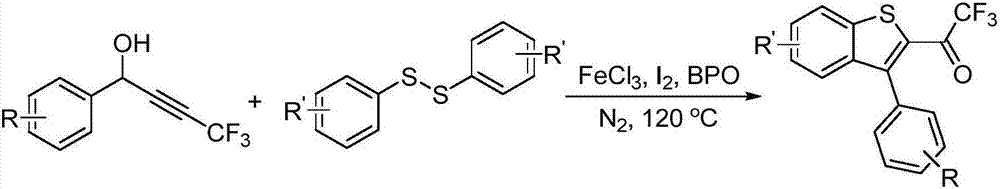
[0023] The preparation method of the trifluoromethylbenzothiophene derivative of the present embodiment comprises the following steps: taking the acetylenic alcohol compound containing fluoromethyl building blocks or its derivatives as the substrate, adding disulfide to the substrate, and The mol ratio of substance and disulfide is 1:0.6; Then take nitromethane solution as solvent dissolution, and in iodine simple substance, the amount of iodine simple substance is 2.0 equivalent; And in iron catalyst FeCl 3 , Benzoyl peroxide nitrogen atmosphere, the preferred FeCl 3 The molar percentage of benzoyl peroxide is 20%, and the molar percentage of benzoyl peroxide is 10%; Through 120 ℃ heating reaction 24h, make crude product, its chemical reaction formula is as follows:
[0024]
[0025] Wherein, R in the general formula of the substrate is one of methyl, tert-butyl, fluorine, chlorine, bromine, benzene, and hydrogen; R' is one of methyl, fluorine, chlorine, and hydrogen.
[...
specific Embodiment 1
[0032] Specific embodiment one: 20.0 mg (0.1 mmol) 4,4,4-trifluoro-1-phenyl-2-butyn-1-alcohol, 13.1 mg (0.06 mmol) diphenyl disulfide, 3.1 mg ( 0.02mmol) FeCl 3 , 2.5 mg (0.01 mmol) of benzoyl peroxide, 50.8 mg (0.2 mmol) of iodine in the reaction test tube, and then 2 mL of CH 3 NO 2 , heated at 120°C for 24 hours under a nitrogen atmosphere, cooled after the reaction, filtered, and the filtrate was rotary evaporated to remove excess iodine and solvent, and the residue was chromatographed on a silica gel column, washed with a mixture of petroleum ether and ethyl acetate, and TLC Detect, combine the effluents containing the product, distill off the solvent with a rotary evaporator, and dry in vacuo to obtain 22.0 mg of 2-trifluoroacetyl-3-phenylbenzothiophene as a yellow liquid with a yield of 72%. 1 H NMR (500MHz, CDCl 3 )δ7.84(d,J=8.0Hz,1H),7.51-7.47(m,2H),7.43-7.40(m,3H),7.33-7.32(m,1H),7.29-7.27(m,2H) ; 13 C NMR (125MHz, CDCl 3 )δ175.6(q,J C-F =37.1Hz), 148.6, 141.9...
specific Embodiment 2
[0034] Specific example two: 21.4 mg (0.1 mmol) 4,4,4-trifluoro-1-(4-tolyl)-2-butyn-1-alcohol, 13.1 mg (0.06 mmol) diphenyl disulfide , 3.1 mg (0.02 mmol) FeCl 3 , 2.5 mg (0.01 mmol) of benzoyl peroxide, 50.8 mg (0.2 mmol) of iodine in the reaction test tube, and then 2 mL of CH 3 NO 2 , heated at 120°C for 24 hours under a nitrogen atmosphere, cooled after the reaction, filtered, and the filtrate was rotary evaporated to remove excess iodine and solvent. The residue was subjected to silica gel column chromatography, washed with petroleum ether, and detected by TLC. liquid, the solvent was distilled off by a rotary evaporator, and dried in vacuo to obtain 25.9 mg of 2-trifluoroacetyl-3-(4-methylphenyl)benzothiophene as a yellow liquid, with a yield of 81%. 1 H NMR (500MHz, CDCl 3 )δ7.91(d, J=8.0Hz, 1H), 7.61(d, J=8.5Hz, 1H), 7.56-7.53(m, 1H), 7.40-7.36(m, 1H), 7.31-7.25(m ,4H),2.45(s,3H); 13 C NMR (125MHz, CDCl 3 )δ175.7(q,J C-F =36.9Hz), 148.8, 141.9, 139.7, 138.7, 130...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com