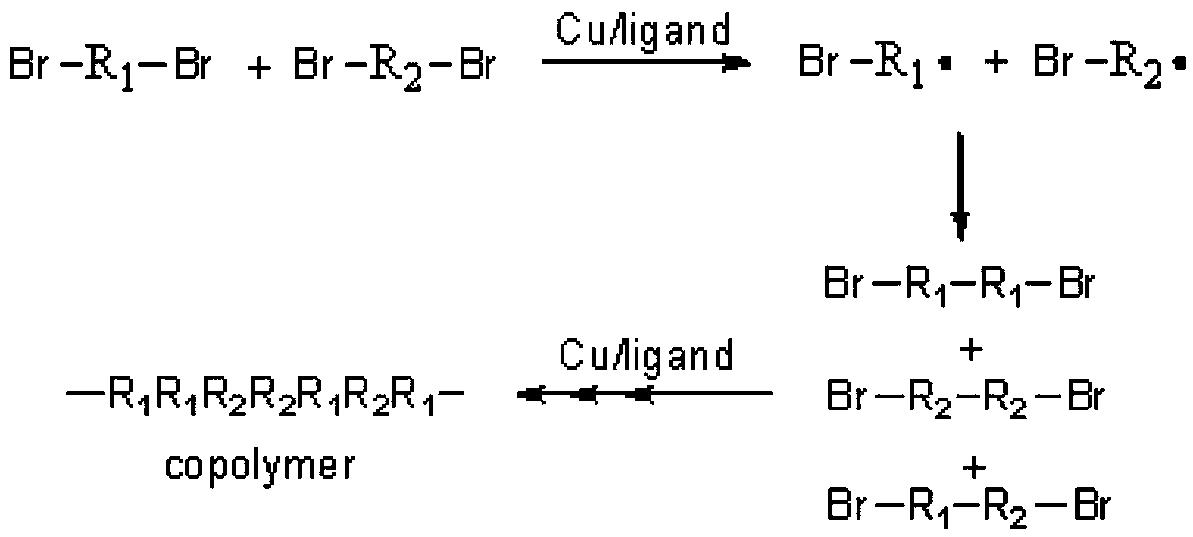
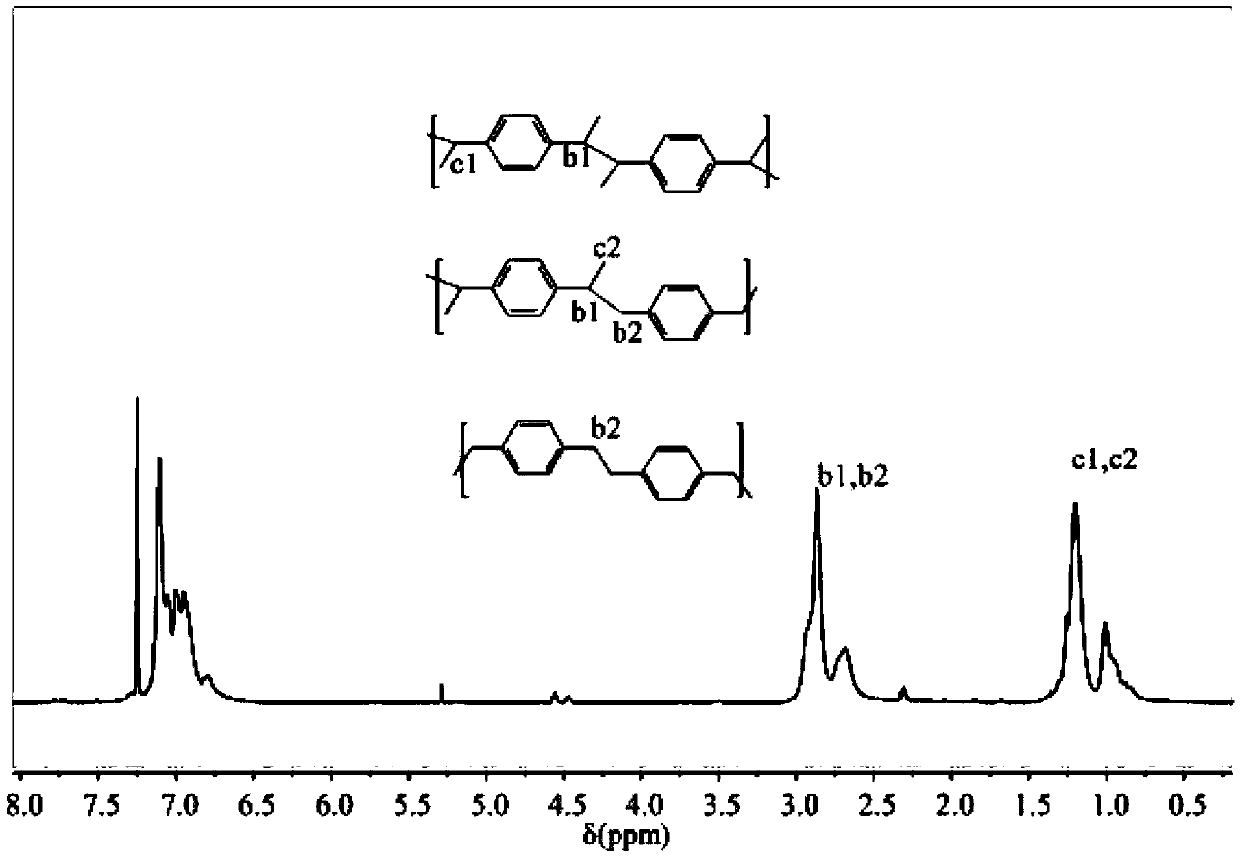
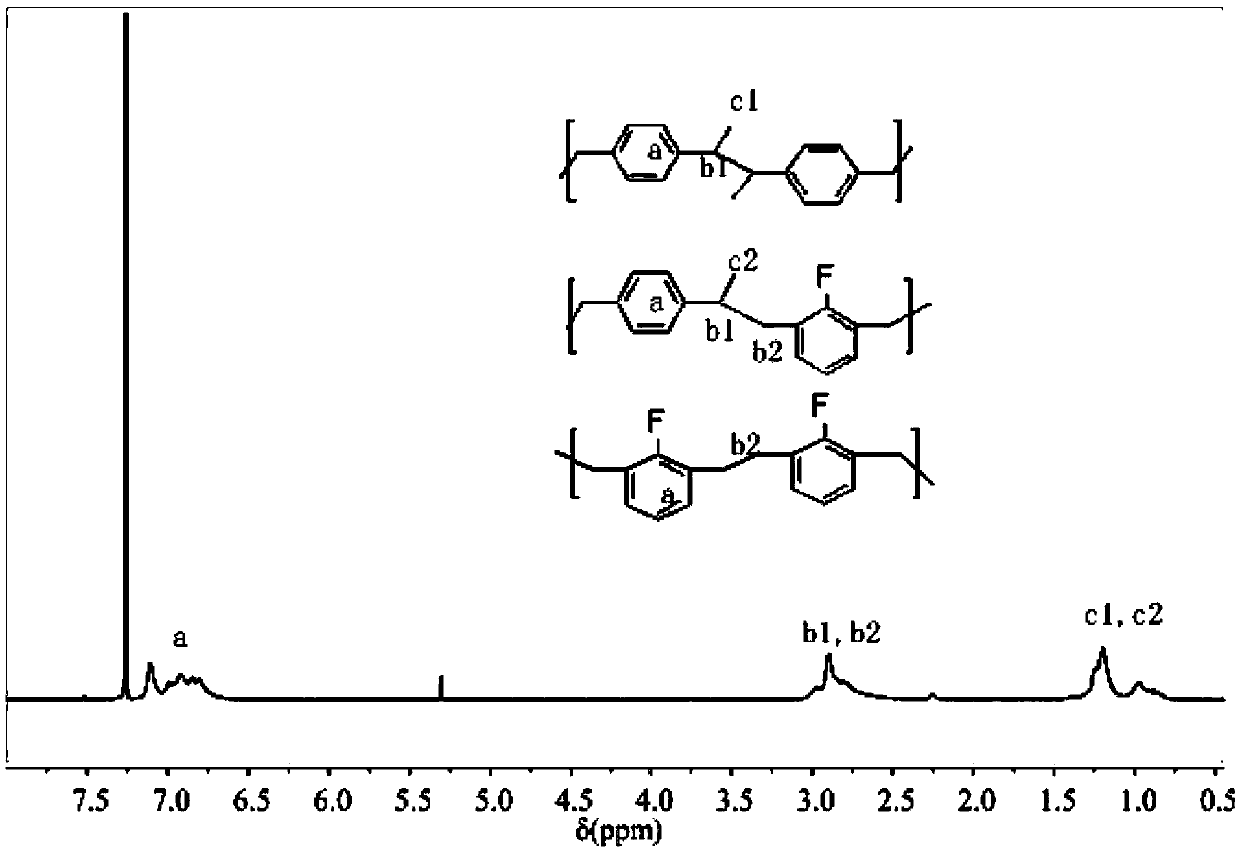
Novel method for preparing copolymer based on co-coupling reaction of two different kinds of carbon free radicals
A coupling reaction and free radical technology, applied in the field of polymer preparation, to achieve the effect of simple synthesis conditions, convenient synthesis, and variable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] (1) Preparation of monomer
[0032] All dibromo monomers can be prepared by existing common synthesis methods, and the molecular structures of the dibromo compounds used in the examples are shown in Table 1 below.
[0033] The molecular structural formulas of eight kinds of dibromocompounds A, B, C, D, E, F, G, H are as follows respectively:
[0034]
[0035] Table 1 Different dibromo compound monomers and their abbreviations
[0036] monomer abbreviation
monomer name
A
1,4-bis(1-bromoethyl)benzene
B
1,4-Dibromomethylbenzene
C
1,3-Dibromomethyl-2-fluorobenzene
D
Methyl 2,5-Dibromomethylbenzoate
E
Methyl 3,5-Dibromomethylbenzoate
F
Bis(4-bromomethylphenyl) ether
G
Bis(4-bromomethylphenyl)sulfone
H
1,4-bis(1-bromo-1-methylethyl)benzene
[0037] (2) Polymerization method
[0038] The measured two kinds of dibromo compounds and ligands, copper powder (also can be...
Embodiment 1
[0043] The synthesis of embodiment 1TPMA
[0044] Dissolve 16.4g of 2-chloromethylpyridine hydrochloride in 40mL of deionized water, cool in an ice bath, slowly add 20mL of 5M NaOH aqueous solution, and the solution turns pink. Add 80 mL of CH containing 5.4 g of 2-(aminomethyl)pyridine 2 Cl 2 solution, warmed to room temperature. Add 20 mL of 5M NaOH aqueous solution with a micro-syringe, and drop it in 50 hours. Stop the reaction, wash the organic phase with 3×10mL15% NaOH aqueous solution, combine the organic phases, anhydrous MgSO 4 Dry, filter and concentrate. The product was extracted with diethyl ether in a boiling state, the insoluble matter was removed, cooled, the product was crystallized in diethyl ether, and filtered. The recrystallization was continued for 3 times to obtain pale yellow needle crystals with a yield of 37%. 1 HNMR (400MHz, CDCl 3 ): 8.54-8.53 (d, 3H), 7.67-7.64 (t, 3H), 7.60-7.58 (d, 3H), 7.16-7.13 (t, 3H), 3.89 (s, 6H).
Embodiment 2
[0045] The synthesis of embodiment 2 monomer A
[0046] Add 11.7g of N-bromosuccinimide, 0.3g of benzoyl peroxide, 4.7mL of 1,4-diethylbenzene, and 50mL of carbon tetrachloride into a 100mL three-necked flask in turn, blow nitrogen gas for 10 minutes, and reflux for 1.5 hours . Stop the reaction, filter, and concentrate the filtrate to obtain a light yellow solid. The crude product was recrystallized from n-hexane to obtain white crystals, which were dried in vacuo with a yield of 60%. 1 HNMR (400MHz, CDCl 3 ): 7.41 (s, 4H, ArH), 5.22-5.17 (q, 2H, 2CHBrCH 3 ), 2.04-2.03 (d, 6H, 2CHBrCH 3 ). Elemental analysis results: theoretical value (%): C, 41.13; H, 4.14; measured value (%): C, 40.97; H, 4.12.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com