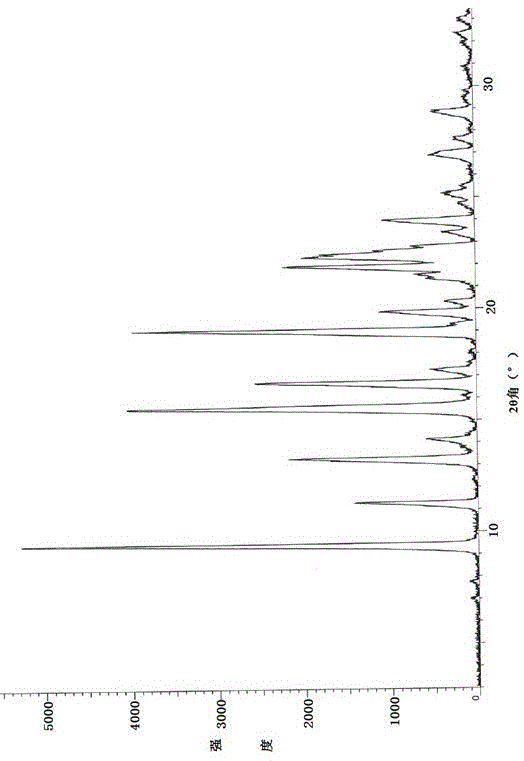
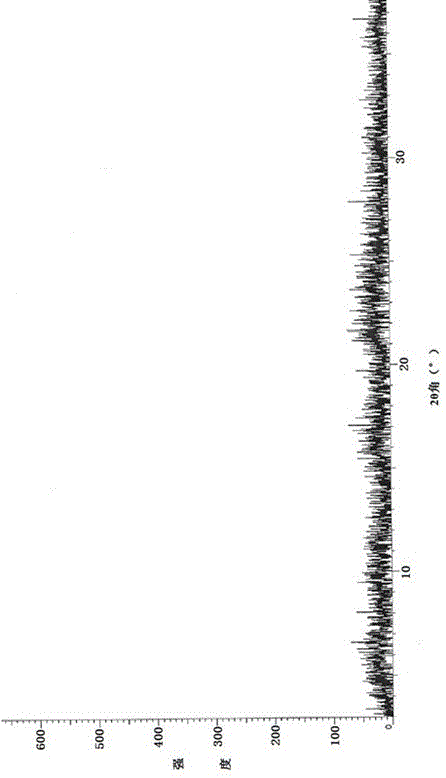
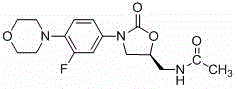
Preparation method of linezolid in type B crystal form
A technology of linezolid and crystal form, applied in the field of preparation of linezolid crystal form B, can solve the problems of low yield, easy moisture absorption and deterioration, unstable linezolid hydrochloride and the like, and achieves good reproducibility , the effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Put 10.0g of linezolid crude product, with a chemical purity of 96.5%, and 500ml of toluene in a reaction bottle, stir and heat to 70°C~80°C, the solid dissolves, and hydrogen chloride gas is introduced, and the reaction liquid immediately turns into white turbidity. When the pH of the reaction liquid = 2.0~2.5, stop feeding the hydrogen chloride gas, cool the reaction solution to room temperature naturally, continue to stir for 2 hours, filter and wash. The filter cake was vacuum-dried at 40°C to 50°C to obtain amorphous linezolid hydrochloride.
[0041] Transfer the amorphous linezolid hydrochloride to the reaction bottle, add 40 times the volume of water, stir and heat to 40°C, add 10% potassium carbonate solution dropwise, adjust the pH of the reaction solution to 9~10, stir and crystallize, Naturally cool to room temperature, filter and wash with water. The resulting filter cake was vacuum-dried at 40°C to 50°C for 4 to 6 hours to obtain 9.1 g of linezolid crystal...
Embodiment 2
[0043] Put 50g of linezolid crude product, with a chemical purity of 96.5%, and 2.5L of ethanol in a reaction flask, stir and heat to 60°C~70°C, the solid dissolves, and hydrogen chloride gas is introduced, and the reaction liquid immediately becomes white turbid. When the pH of the reaction liquid =1.5~2.0, stop feeding the hydrogen chloride gas, cool the reaction solution to room temperature naturally, continue to stir for 2 hours, filter and wash. The filter cake was vacuum-dried at 40°C to 50°C to obtain amorphous linezolid hydrochloride.
[0044]Transfer the amorphous linezolid hydrochloride to the reaction bottle, add 45 times the volume of water, stir and heat to 40°C, add 10% potassium carbonate solution dropwise, adjust the pH of the reaction solution to 9~10, stir and crystallize, Naturally cool to room temperature, filter and wash with water. The resulting filter cake was vacuum-dried at 40°C~50°C for 4~6 hours to obtain 45.6g of Linezolid Form B, with a chemical p...
Embodiment 3
[0045] Embodiment 3 process parameter investigation
[0046] Operation is basically the same as in Example 1, and the results are shown in Table 1, Table 2 and Table 3.
[0047] Table 1. The influence of different solvents and their dosages on the yield, purity and residual solvent of Linezolid Form B.
[0048] NA: not detected
[0049] Table 2. Using toluene as a solvent, investigate the influence of the pH value of the reaction solution on the yield of Linezolid Form B after hydrogen chloride gas is introduced.
[0050]
[0051] Table 3. The influence of the amount of water on the yield, purity and residual solvent of linezolid crystal form B was investigated.
[0052]
[0053] NA: not detected
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com