Preparation method and use of thioctic acid-modified polyethylene glycol-polyaminoacid block copolymer
A technology of lipoic acid and lipoyl group is applied in the field of modified amphiphilic triblock polymers to achieve the effect of overcoming easy leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
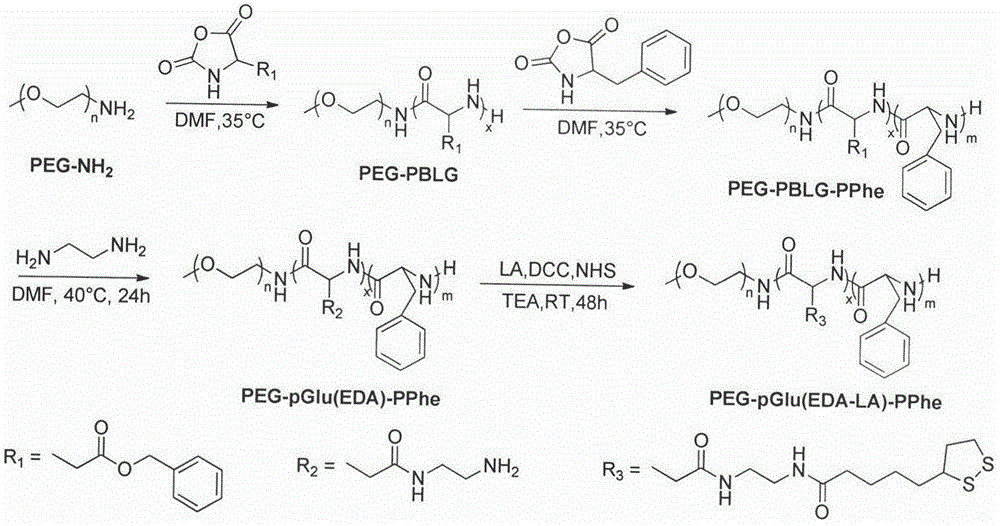
[0036] Embodiment one, synthetic polymer PEG-pGlu(EDA-LA)-PPhe 20 (DP=10, PEG:Mn=5kDa)
[0037] Under nitrogen protection, the CH 3 O-PEG-NH 2 (0.3g, 0.06mmo) was dissolved in dry DMF (3mL), BLG-NCA (189.4mg, 0.72mmol) was added thereto, the system was warmed up to 35°C, and after stirring for 24h, Phe-NCA was added thereto ( 252.1mg, 1.32mmol) and DMF (6mL), continue to stir the reaction at 35°C for 24h. Settling in anhydrous ether gave PEG-PBLG-PPhe triblock polymer.
[0038] Under nitrogen protection, PEG-PBLG-PPhe (0.2 g, 0.182 mmol of benzyl functional group) was dissolved in dry DMF (4 mL) at 40 °C, to which was added distilled ethylenediamine (0.656 g, 10.92 mmol), after stirring and reacting for 36h, 10% acetic acid solution (12mL) was added dropwise to the system, dialyzed 3 times with 0.01M aqueous HCl solution, dialyzed 3 times with distilled water, and freeze-dried to obtain PEG-pGlu(EDA)-PPhe polymer Hydrochloride.
[0039] Under nitrogen protection, PEG-pGl...
Embodiment 2
[0040] Embodiment two, synthetic polymer PEG-pGlu (EDA-LA) 5 -PPhe 20 (DP=5, PEG:Mn=5kDa)
[0041] Under nitrogen protection, the CH 3 O-PEG-NH 2 (0.4g, 0.08mmo) was dissolved in dry DMF (4mL), BLG-NCA (126.2mg, 0.48mmol) was added thereto, the system was warmed up to 35°C, and after stirring for 24h, Phe-NCA was added thereto ( 336.2mg, 1.76mmol) and DMF (8mL), continue to stir the reaction at 35°C for 24h. Settling in anhydrous ether gave PEG-PBLG-PPhe triblock polymer.
[0042] Under nitrogen protection, PEG-PBLG-PPhe (0.3 g, 0.1945 mmol of benzyl functional groups) was dissolved in dry DMF (6 mL) at 40 °C, to which was added distilled ethylenediamine (0.58 g, 9.726 mmol), after stirring and reacting for 36h, 10% acetic acid solution (7.2mL) was added dropwise to the system, dialyzed 3 times with 0.01M aqueous HCl solution, dialyzed 3 times with distilled water, and freeze-dried to obtain PEG-pGlu(EDA)-PPhe polymer substance hydrochloride.
[0043] Under nitrogen pro...
Embodiment 3
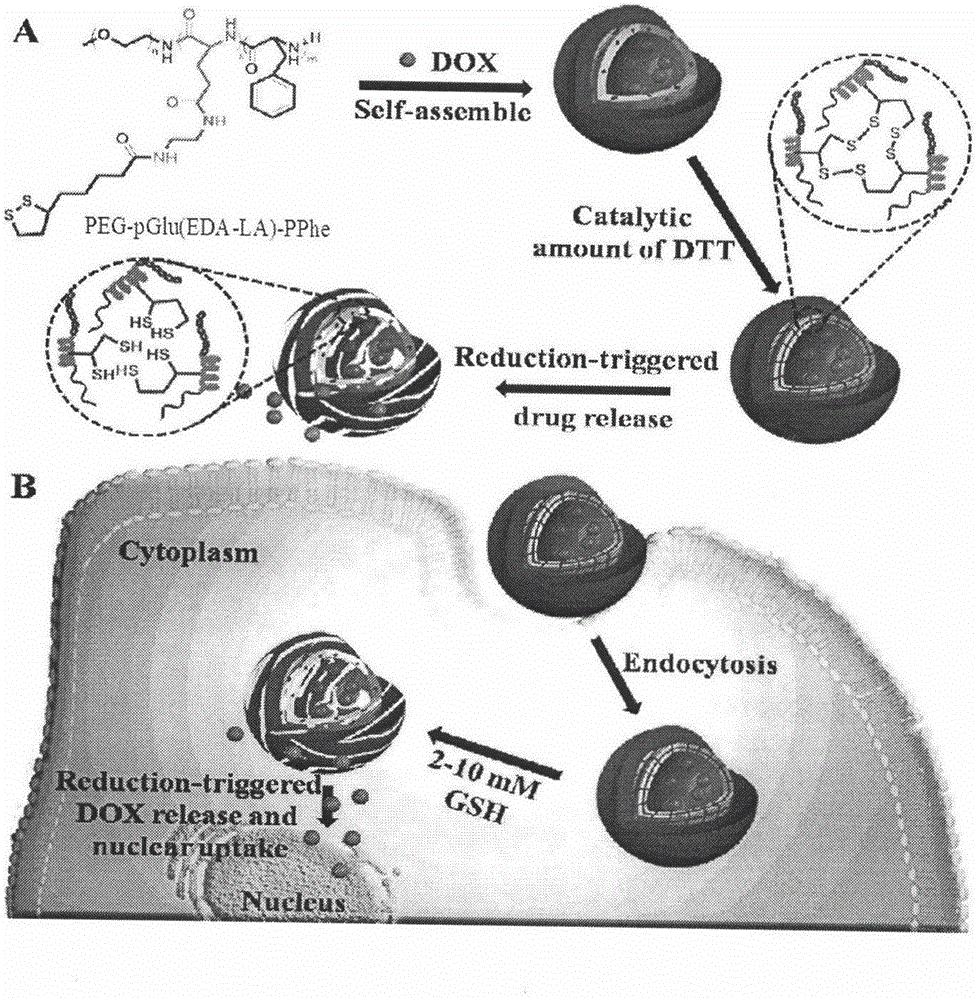
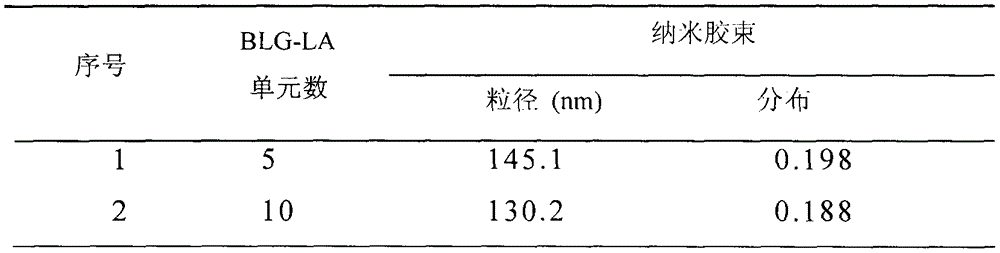
[0044] Embodiment three, PEG-pGlu (EDA-LA) 10 -PPhe 20 (DP=10, PEG: Mn=5kDa) Nanomicelle Preparation
[0045] Polymer PEG-pGlu(EDA-LA) 10 -PPhe nanomicelles were prepared by a dialysis method. The specific process is: 2 mg polymer PEG-pGlu (EDA-LA) 10 - PPhe was dissolved in 1 mL of dimethyl sulfoxide, and 5 mL of deionized water was added dropwise thereto under stirring at 25°C. After the obtained solution was stirred for 1 h, it was put into a pre-prepared dialysis bag (SPECTRA / POR, MWCO: 3500), and dialyzed with deionized water for 24 h. The nanomicelle is 130.2 nanometers, and the particle size distribution is 0.188.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com