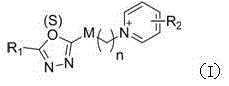
Pyridine salt compound containing 1,3,4-oxadiazolyl (thiadiazolyl) and preparation method and application thereof
A technology for salt compounds and pyridine, which is applied in the field of pyridine salt compounds and their preparation, and can solve the problems of reducing the bacteriostatic activity of the compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
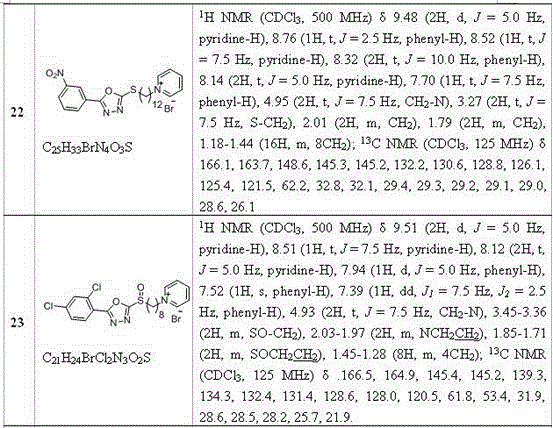
[0022] Example 1 of the present invention: target compound 10-[5-(2,4-dichlorophenyl)-2-mercapto-1,3,4-oxadiazole]decyl ether-1-pyridinium bromide preparation
[0023] Dissolve 0.53g (1.1mmol) 2-(10-bromodecyl)mercapto-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole in 5mL pyridine solvent and react at 50°C Stop the reaction after 6h, remove the solvent, and wash with ether to obtain 0.54 g of white solid, yield 87.1%, melting point: 96-98°C.
[0024] The synthesis of oxygen ether and thioether target compounds refers to Example 1.
Embodiment 2
[0025] Example 2 of the present invention: target compound 10-[2-sulfoxide-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole]decyl-1-pyridinium bromide preparation of
[0026] Dissolve 0.45g of 2-(10-bromodecyl)sulfoxide-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole in 5mL of pyridine, react at 50°C for 6h, and remove the solvent. Purified by column chromatography to obtain 0.42 g of light yellow solid with a yield of 80.2% and a melting point of 84-86°C.
[0027] The synthesis of sulfoxide and sulfone target compounds refers to Example 2.
[0028] The structure, H NMR spectrum and carbon spectrum data of some synthesized pyridinium salt compounds containing 1,3,4-oxa(thia)diazolyl are shown in Table 1, and their physical and chemical properties are shown in Table 2.
[0029]
[0030]
[0031]
[0032]
[0033]
[0034]
[0035]
[0036]
[0037]
[0038]
[0039]
[0040]
[0041]
[0042]
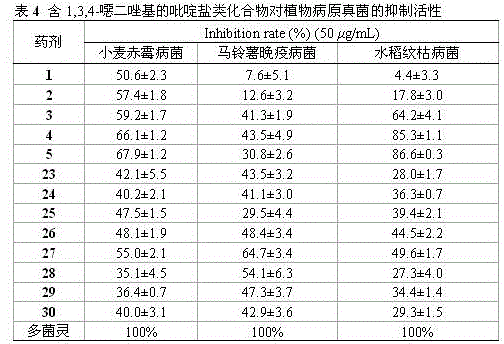
[0043] Pharmacological Example 1: EC 50 (median effectiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com