Single-component phosphor-nitrogen double-ring cage-shaped macromolecular intumescent flame retardant as well as preparation method and application thereof
A technology of intumescent flame retardants and macromolecules, which is applied in the field of one-component phosphorus-nitrogen bicyclic cage macromolecular intumescent flame retardants and its preparation, and can solve the problems of poor compatibility and easy migration of polymer matrix , to achieve good compatibility, overcome easy migration, and save costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
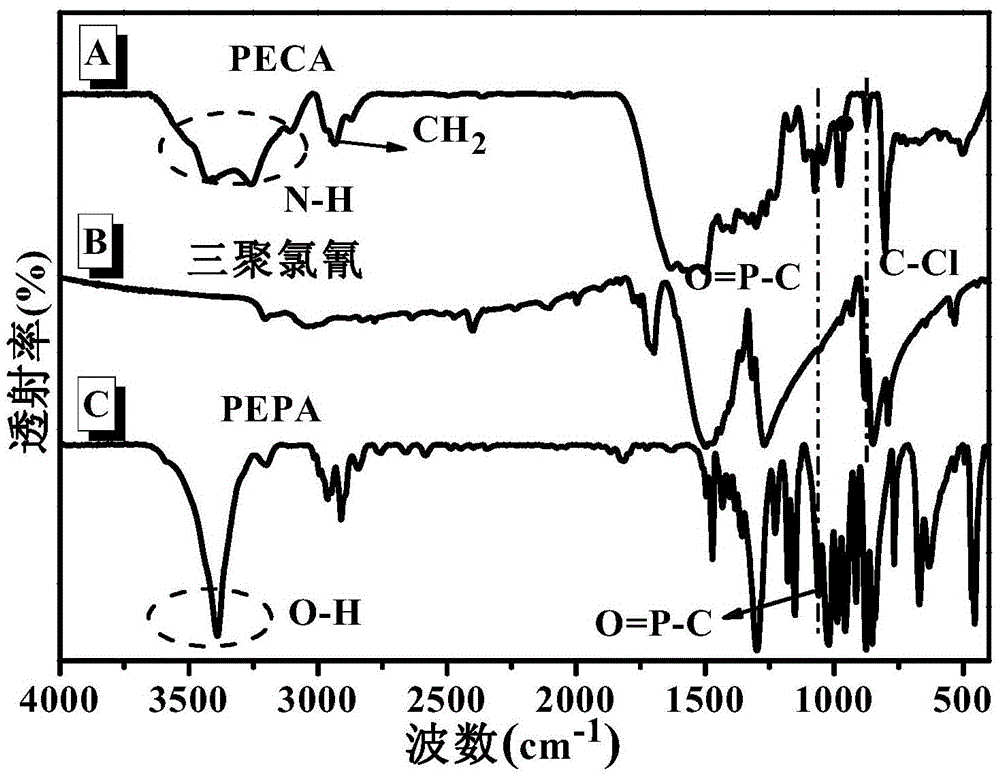
[0035] 1) Synthesis of 1-oxo-4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane (PEPA): add 150mL di Hexane and 34.04g (0.25mol) of pentaerythritol, heated to 95 ° C, mechanically stirred for 30min; 38.33g (0.25mol) of phosphorus oxychloride dissolved in 50mL of dioxane, dropwise added to the flask within 1h, and Dissolve 30.00g (0.75mol) of sodium hydroxide in 120mL of deionized water to prepare an acid-binding agent, and add it dropwise to the flask at the same time, and continue to react for 4 hours after the drop is completed; after the reaction is completed, let it stand for cooling, concentrate and filter the mixture, Wash with dioxane and ethanol successively, and then dry to obtain white solid powder PEPA.
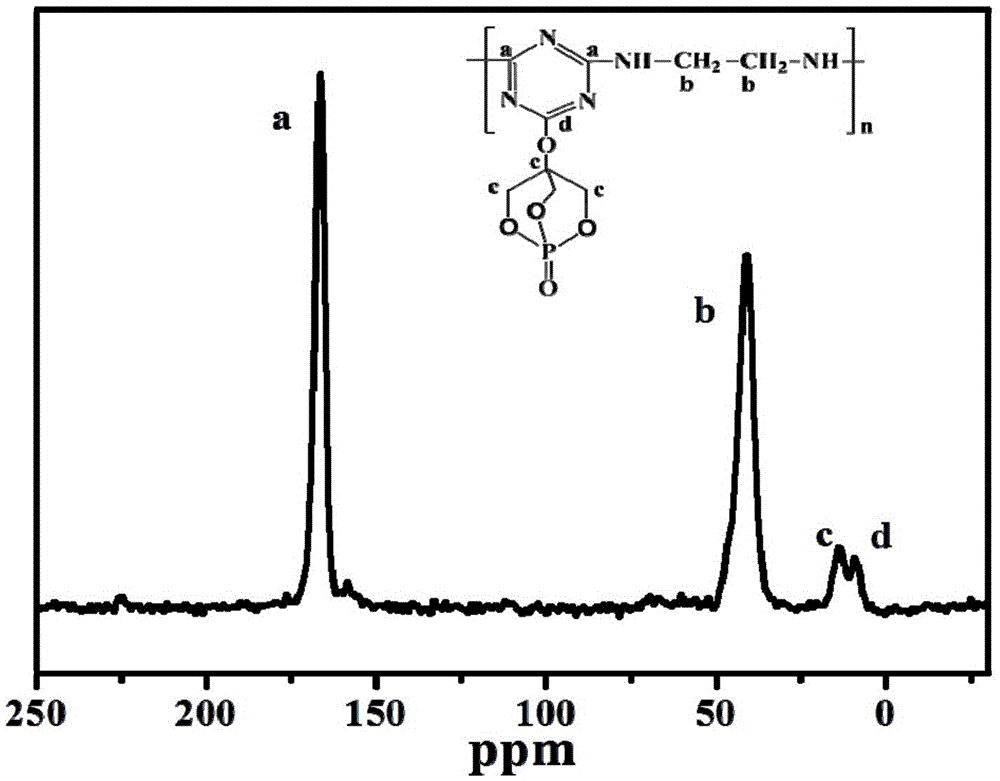
[0036]2) Synthesis of one-component phosphorus-nitrogen bicyclic cage macromolecular expansion flame retardant (PECA): Add 200mL dioxane, 18.44g (0.1mol ) cyanuric chloride and 18.01g (0.1mol) PEPA, mechanically stirred for 30min, 4g (0.1mol) sodium hydrox...
Embodiment 2
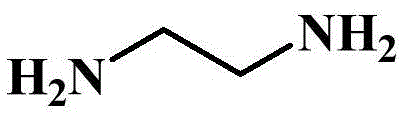
[0041] The difference between this example and Example 1 is that the reaction temperature in the 50°C reaction stage in step 2) is increased to 55°C, and 3.01g (0.05mol) of 1,2-ethylenediamine is replaced by 5.41g (0.05mol) For 1,4-phenylenediamine, the dropping time is extended to 2.5 hours, and the reaction time is extended to 4 hours after the dropping; the reaction temperature in the 95°C reaction stage is raised to 100°C, and 3.01g (0.05mol) of 1,2-ethylenediamine is replaced 5.41g (0.05mol) of 1,4-phenylenediamine was obtained, the amount of sodium hydroxide was increased to 4.4g (0.11mol), the dropping time was extended to 2.5h, and the reaction time was extended to 6h after the dropping. The obtained product is a white solid powder, and its structural formula is shown in the following formula. The benzene ring introduced by phenylenediamine is also an important carbon source, and has good thermal stability, and the resulting carbon layer has a stable structure. The av...
Embodiment 3
[0044] The difference between this example and Example 1 is that the reaction temperature in the 50°C reaction stage in step 2) is raised to 60°C, and 3.01g (0.05mol) of 1,2-ethylenediamine is replaced by 9.91g (0.05mol) For 4,4'-diaminodiphenylmethane, the dropwise addition time was extended to 3h, and the reaction time was extended to 5h after the dropwise completion; the reaction temperature was raised to 105°C in the 95°C reaction stage, and 3.01g (0.05mol) of 1,2-ethanedi The amine was replaced by 9.91g (0.05mol) of 4,4'-diaminodiphenylmethane, the amount of sodium hydroxide was increased to 4.4g (0.11mol), the dropping time was extended to 3h, and the reaction time was extended to 7h after the dropping. The obtained product is a white solid powder, and its structural formula is shown in the following formula. The average molecular weight measured by gel permeation chromatography (using dimethyl sulfoxide as a solvent and polystyrene with a narrow molecular weight distrib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| impact strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com