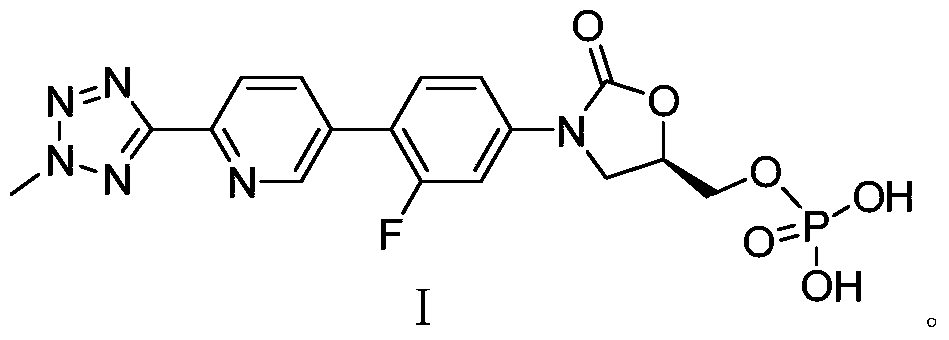
A kind of preparation method of bacterial protein synthesis inhibitor
A solvent and compound technology, which is used in the preparation of bacterial protein synthesis inhibitors and the preparation of tedizolid phosphate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
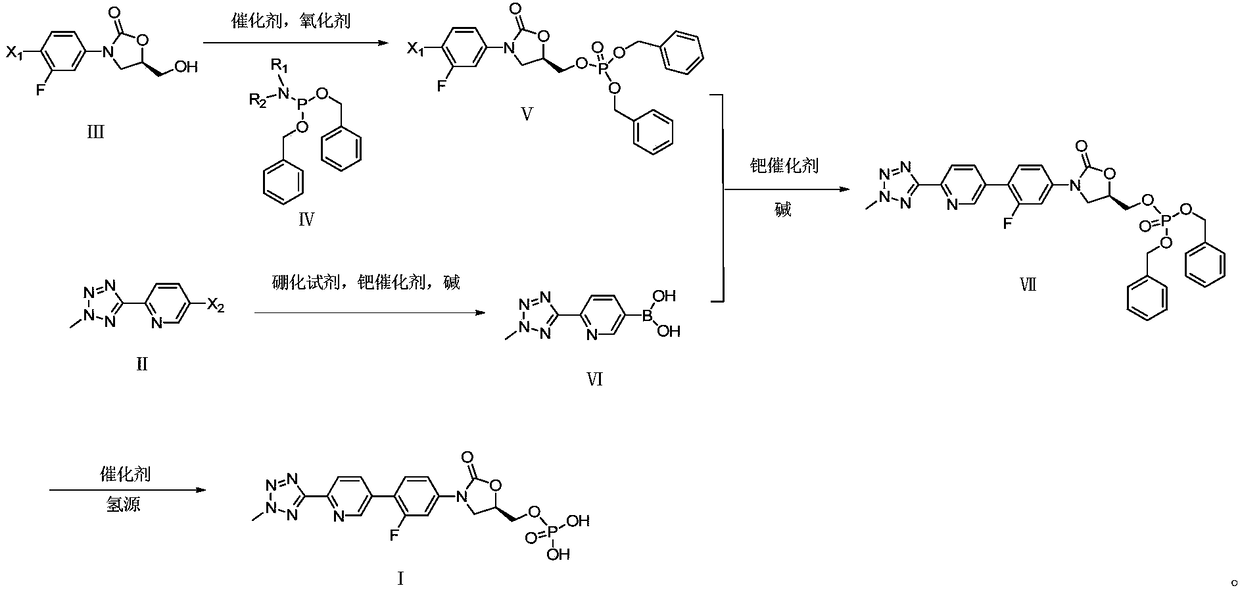
[0077] Preparation of Example 1 (R)-[3-(4-bromo-3-fluorophenyl)-2-oxo-5-oxazolidinyl]methyl phosphate bis(benzyl ester) (compound of formula VIII)
[0078] Add dichloromethane (50ml), 1H-tetrazolium (7.24g, 103.41mmol) and (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazole in 250ml there-necked flask Alkan-2-one (10.0g, 34.47mmol), temperature controlled below 30°C, add dropwise dibenzyl diisopropylamino phosphite (23.81g, 68.94mmol), keep the temperature at 25-30°C for 30min, cool down to 0-10°C, add 85% m-chloroperoxybenzoic acid (9.8g, 48.26mmol), and react at 5-10°C for 30min.
[0079] The reaction solution was sequentially washed with saturated NaHCO 3 Washed twice, washed once with saturated NaCl solution, anhydrous NaCl 2 SO 4 Drying, filtration, concentration in vacuo and purification by column chromatography afforded 17.11 g of the title compound in 90.2% yield and 99.24% purity by HPLC (area normalized method).
[0080] 1 H NMR (500MHz, DMSO-d6): δ7.6892(m,1...
Embodiment 2
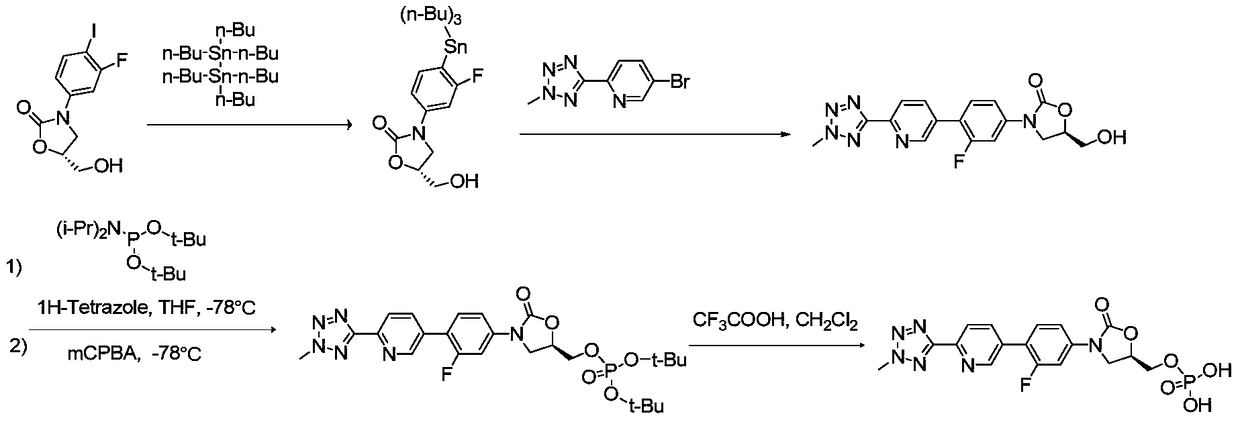
[0083] Preparation of Example 2B-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl]boronic acid (compound of formula VI)
[0084] DMSO (100ml), 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (10.0g, 41.66mmol), pinacol diborate (12.69g, 49.99mmol), [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (1.7g, 2.08mmol) and potassium acetate (16.35g, 166.64mmol), N 2 Under protection, the temperature was raised to 80°C, and the reaction was carried out for 3h. Dichloromethane / water extraction, the separated organic phase was washed with saturated NaCl solution, anhydrous Na 2 SO 4 Dehydration, filtration, vacuum concentration and purification by column chromatography afforded 8.11 g of solid with a yield of 95.0% and a purity of 98.2% by HPLC (area normalization method).
[0085] 1 H NMR (500 MHz, DMSO-d6): δ 8.9245 (s, 1H), 8.2169 (dd, 1H), 8.1549 (dd, 1H), 4.4811 (s, 3H).
[0086] 13 C NMR (125 MHz, DMSO-d6): δ 163.901, 154.885, 148.290, 143.277, 121.612,...
Embodiment 3
[0088] Example 3 (R)-[3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-2-oxo-5-oxazole Preparation of Alkyl]methyl Phosphate Bis(benzyl Ester) (Compound of Formula VII)
[0089] Add 1,4-dioxane (60ml), compound of formula VIII (17.0g, 30.89mmol), compound of formula VI (6.97g, 33.98mmol), [1,1'-bis(diphenyl Phosphine) ferrocene] dichloropalladium dichloromethane complex (0.51g, 0.62mmol), sodium carbonate aqueous solution (30ml, containing 10.8g sodium carbonate, 101.94mmol), N 2 Under protection, the temperature was raised to 70°C, reacted for 3h, added dichloromethane for extraction, and the separated organic phase was washed with saturated NaCl solution, anhydrous NaCl 2 SO 4 Dehydration, filtration, vacuum concentration and purification by column chromatography yielded 15.31 g of a solid with a yield of 78.6% and a purity of 98.43% by HPLC (area normalization method).
[0090] 1 H NMR (500MHz, DMSO-d6): δ8.9318(s,1H),8.241(m,1H),8.1938(m,1H),7.7457(t,1H),7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com