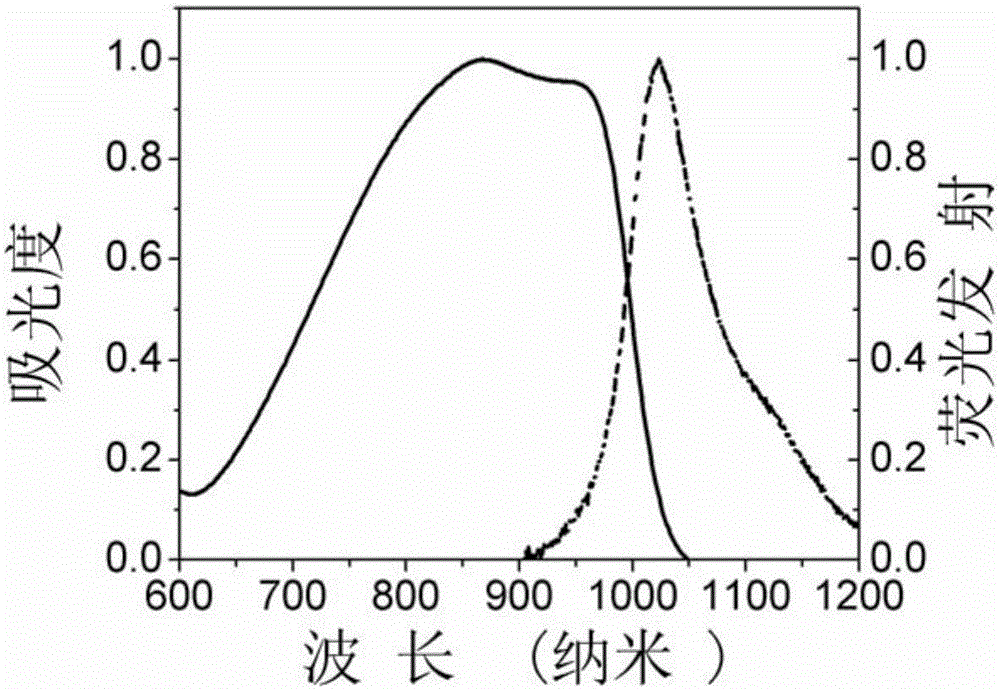
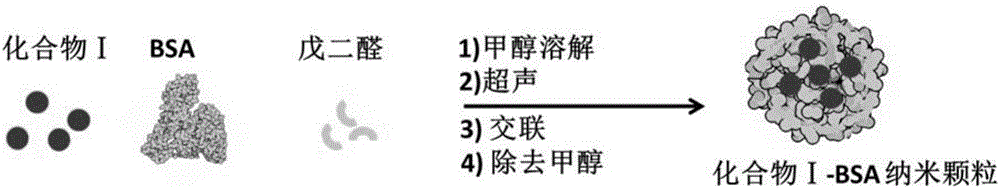
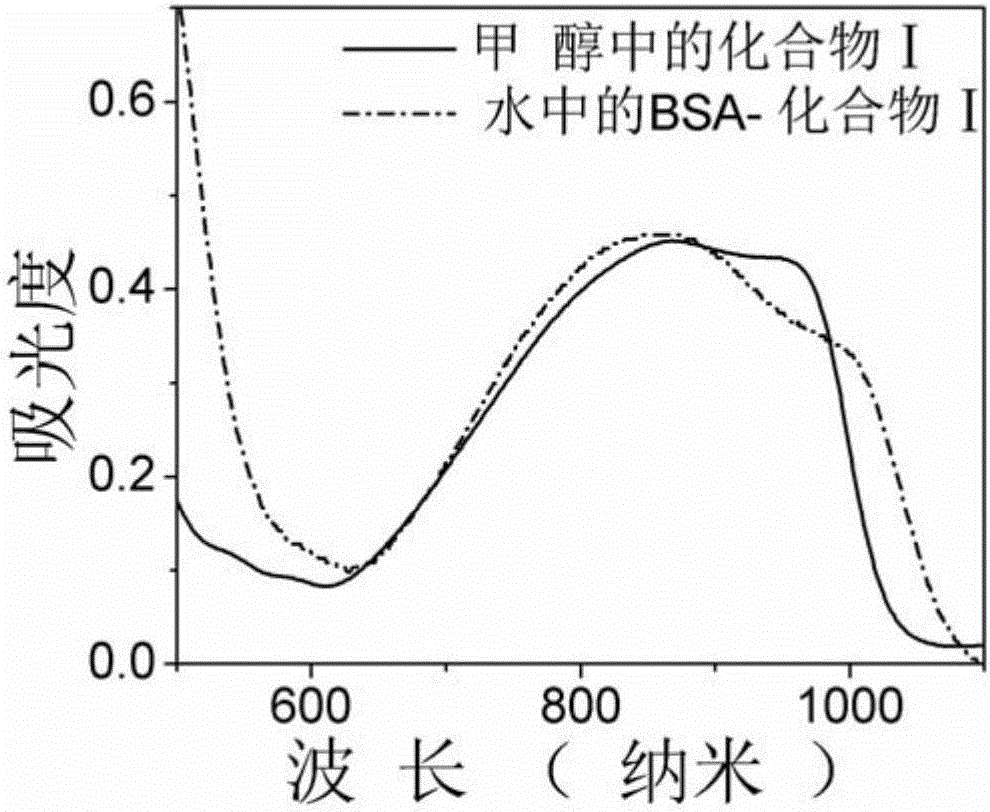
A kind of asymmetric near-infrared cyanine dye and its preparation method and application
An infrared cyanine and asymmetric technology, applied in the field of biomedical materials, can solve the problems of non-degradability and achieve the effects of large conjugated system, good tumor inhibition effect, and good photothermal conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of the intermediate shown in embodiment 1, formula II
[0062]
[0063] In 20mL of dry DMF, slowly add 18.5mL (200mmol) phosphorus oxychloride, heat to 50°C, slowly add 5g (51mmol) cyclohexanone after 30min, then raise the temperature to 60°C, and react for 8h. After the reaction, cool to room temperature with ice water, and then pour into 100 g of crushed ice four times. Stir at room temperature and stand overnight to obtain a yellow solid, which is filtered and recrystallized from dichloromethane to obtain the intermediate represented by formula II as needle-shaped yellow crystals.
[0064] 1 H NMR (600MHz, DMSO-D6) δ10.06(s, 1H), 4.06(s, 1H), 3.25(t, 4H), 2.63(m, 2H).
[0065] Confirmed that the structure is correct.
Embodiment 2
[0066] The synthesis of the intermediate shown in embodiment 2, formula III
[0067]
[0068] Add 2.0g (20mmol) cyclohexanone into 10mL concentrated sulfuric acid with stirring and cool to 0°C, weigh 3.13g (10mmol) 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid and add to the above reaction solution, stirred and heated at 90°C for 2 h, after the reaction was completed and cooled to room temperature, poured into 200 g of crushed ice, added 4 mL of perchloric acid, filtered, washed with cold water, and dried in vacuum to obtain the intermediate shown in formula III, which was directly purified without purification for the next reaction.
[0069] 1 H NMR (600MHz, DMSO-D6) δ8.17(d, J=7.9Hz, 1H), 7.83(t, J=7.5Hz, 1H), 7.74(t, J=7.7Hz, 1H), 7.29(t ,J=9.2Hz,2H),7.17(d,J=16.1Hz,1H),7.00(d,J=9.6Hz,1H),3.63(d,J=6.9Hz,4H),3.18–2.98(m ,2H),2.44(d,J=26.4Hz,2H),2.12(dt,J=16.4,13.3Hz,2H),1.84(s,2H),1.67(ddd,J=26.1,12.8,6.9Hz, 2H), 1.17(t, J=6.7Hz, 6H), 0.98(t, J=7.0Hz, 1H).
[0070]...
Embodiment 3
[0071] The synthesis of the intermediate shown in embodiment 3, formula IV
[0072]
[0073] Under the protection of nitrogen, the raw material 2,3,3-trimethyl-4,5-benzo-3H-indole (4.0g, 19.1mmol) was dissolved in dry acetonitrile, and ethyl iodide (14.9g, 95.5 mmol), heated and stirred at 85°C for 10 h. After the reaction solution was cooled to room temperature, the purple solid intermediate represented by formula IV was obtained by filtration, washed with acetone, dried under vacuum, and directly used in the next reaction without further purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com