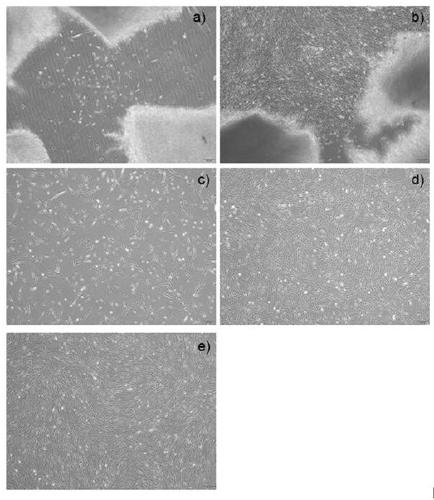
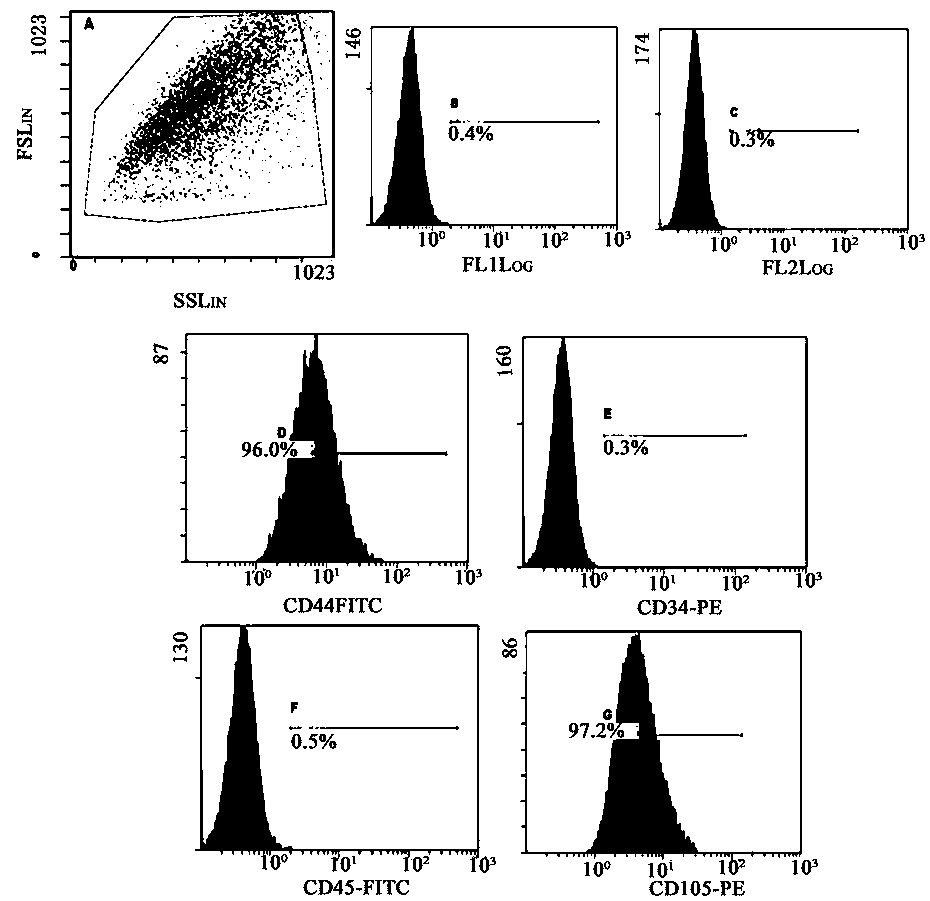
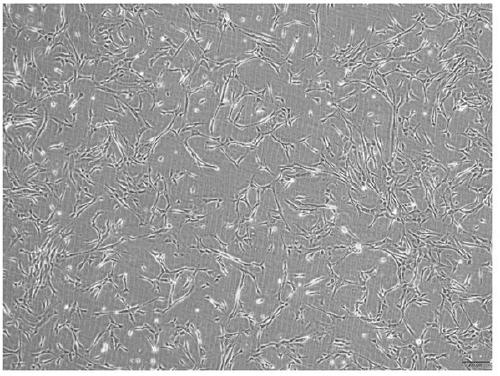
A method to obtain safe and effective umbilical cord mesenchymal stem cells
A technology for stem cells and umbilical cord, applied in the field of umbilical cord mesenchymal stem cells, can solve the problems of low separation efficiency, long cycle, insufficient safety, etc., and achieve the effects of improving separation efficiency, ensuring safety, and shortening culture period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1 Umbilical cord preparation:
[0049] 1) In a sterile environment, the umbilical cord is collected during the third stage of labor, and the residual blood of the umbilical cord and vein is drained and ligated; the collected umbilical cord is disinfected and placed in a storage bottle with storage liquid, and sent to the laboratory for further investigation. Prepared separately. The storage solution is 40ml of α-MEM, and gentamicin 100U / ml, penicillin 50mg / ml, and amphotericin B 5ug / ml are added. Before collection, ensure that the five tests of the pregnant woman's virus are negative and the ALT test is qualified. The transportation temperature is 4°C, and the transportation time does not exceed 24 hours.
[0050] 2) The umbilical cord bottle is wiped with alcohol and placed in a biosafety cabinet.
[0051] 3) at 150cm 2 In the cell culture dish, wash the umbilical cord 3 times with washing solution, cut off 1 cm at both ends of the umbilical cord with pointed strai...
Embodiment 2
[0078] 1 Umbilical cord preparation:
[0079] 1) In a sterile environment, the umbilical cord is collected during the third stage of labor, and the residual blood of the umbilical cord and vein is drained and ligated; the collected umbilical cord is disinfected and placed in a storage bottle with storage liquid, and sent to the laboratory for further investigation. Prepared separately. The storage solution is 40ml of α-MEM, and gentamicin 100U / ml, penicillin 50mg / ml, and amphotericin B 5ug / ml are added. Before collection, ensure that the five tests of the pregnant woman's virus are negative and the ALT test is qualified. The transportation temperature is 4°C, and the transportation time does not exceed 24 hours.
[0080] 2) The umbilical cord bottle is wiped with alcohol and placed in a biosafety cabinet.
[0081] 3) at 150cm 2 In the cell culture dish, wash the umbilical cord 3 times with washing solution, cut off 1 cm at both ends of the umbilical cord with pointed strai...
Embodiment 3
[0108] 1 Umbilical cord preparation:
[0109] 1) In a sterile environment, the umbilical cord is collected during the third stage of labor, and the residual blood of the umbilical cord and vein is drained and ligated; the collected umbilical cord is disinfected and placed in a storage bottle with storage liquid, and sent to the laboratory for further investigation. Prepared separately. The storage solution is 40ml of α-MEM, and gentamicin 100U / ml, penicillin 50mg / ml, and amphotericin B 5ug / ml are added. Before collection, ensure that the five tests of the pregnant woman's virus are negative and the ALT test is qualified. The transportation temperature is 4°C, and the transportation time does not exceed 24 hours.
[0110] 2) The umbilical cord bottle is wiped with alcohol and placed in a biosafety cabinet.
[0111] 3) at 150cm 2 In the cell culture dish, wash the umbilical cord 3 times with washing solution, cut off 1 cm at both ends of the umbilical cord with pointed strai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com