Novel selection vectors and methods of selecting eukaryotic host cells
A technology of host cells and vectors, applied in the direction of nucleic acid vectors, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of weak transport affinity and inactivation of folic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0171] According to another embodiment, the expression cassette comprises at least
[0172] (i) a polynucleotide encoding the polypeptide of interest,
[0173] (ii) includes a 5' splice donor site and a 3' splice acceptor site, and an intron including an in-frame translation stop codon and polyadenylation signal, and
[0174] (iii) a polynucleotide encoding a membrane anchor and / or a membrane anchor signal downstream of said intron.
[0175] This expression cassette design has the effect that at least 2 different mature mRNAs (mRNA-POI) and (mRNA-POI-ANCHOR) are obtained from the expression cassette through transcription and transcript processing. mRNA-POI translation produces the target polypeptide. mRNA-POI-ANCHOR translation produces a fusion polypeptide containing the polypeptide of interest (POI) and the membrane anchor. Thus, the fusion polypeptide is again displayed on the cell surface, and cells displaying high levels of the membrane-anchored fusion polypeptide can ...
Embodiment 1
[0321] Example 1: Single transfection
[0322] For single transfection, wild-type human folate receptor alpha (vector: V-wtFRalpha) or mutant human folate receptor alpha (vector: V-mutFRalpha) was introduced into CHO cells as a selectable marker. This experiment was used to analyze the function of a folate receptor selection system which, in contrast to the DHFR / MTX-system, is not based on a toxic inhibition of cell growth, but on growth inhibition by loss of folic acid in the medium. Folic acid is the oxidized form of vitamin B9. The reduced form of folic acid, tetrahydrofolate (THF), is biologically active. Intracellular folate is reduced to its biologically active form tetrahydrofolate (THF) by dihydrofolate reductase (DHFR) via dihydrofolate (DHF) in a NADPH-dependent reaction. Folate uptake is essential for mammalian cells to maintain cell growth and cell proliferation.
[0323] Only cells that integrate the transfection vector into the genome and express wild-type fol...
Embodiment 2
[0339] Example 2: Determination of antibody production rate
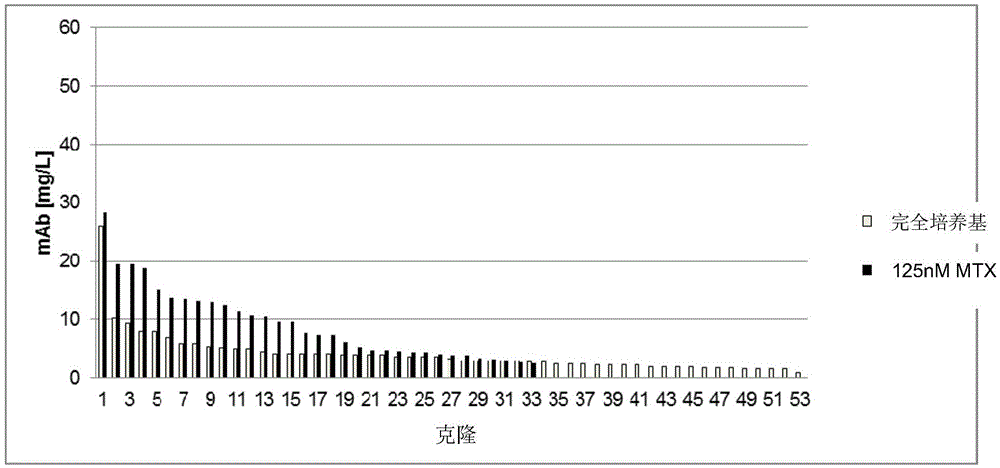
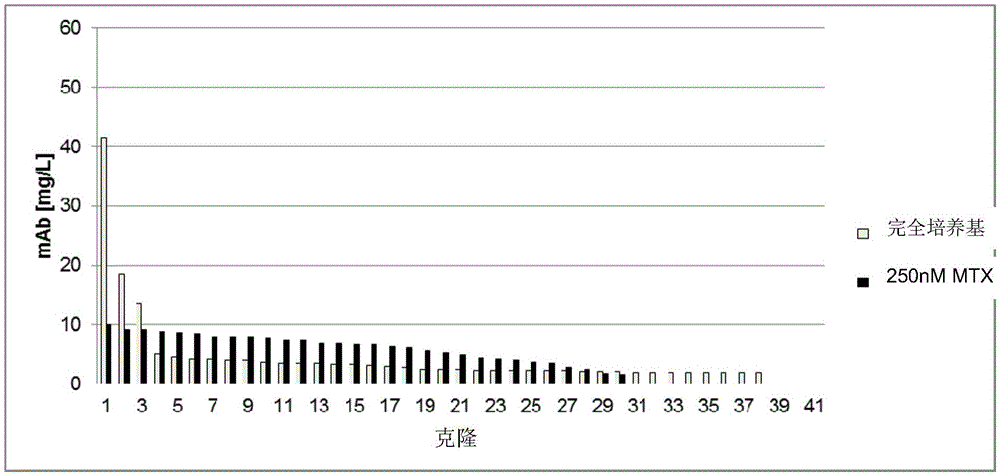
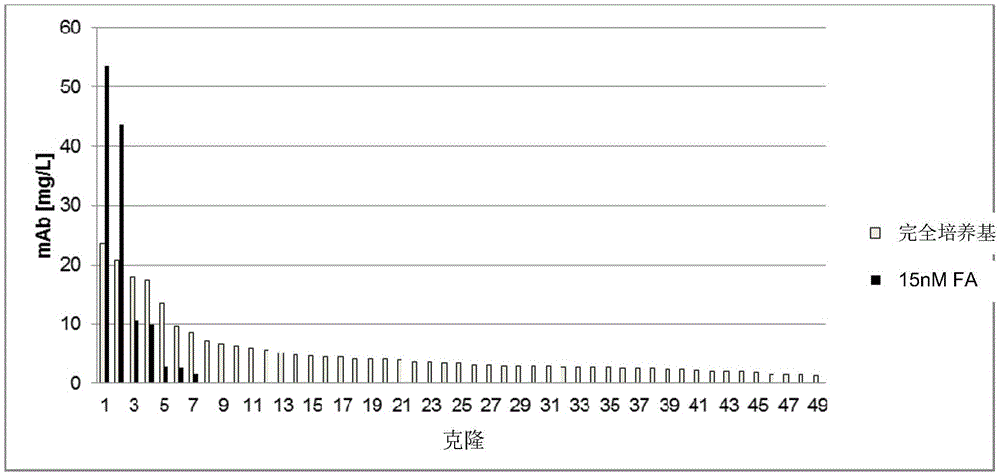
[0340]To analyze the success of the transfection and selection based on the expression of the gene of interest (here the reference antibody), the cells obtained (i.e. selected according to Example 1) were batch cultured in shake flasks for 13 days to determine the cell yield after selection . Cells have greater than 90% viability. On day 13, the antibody concentration of the culture supernatant was determined by protein A affinity chromatography [mg / L]. The results are shown in Table 4.
[0341] Table 4: Antibody concentration (mAb) in batch culture supernatant (mg / L)
[0342]
[0343]
[0344] Antibodies expressed from the introduced expression vectors can be detected in all cell populations. Since small amounts of antibody were also detected in the pool of cells not transfected with DNA, only values greater than 9 mg / l were determined to be significant. The reference population in 125nMMTX (standard D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com