Preparation method of silver coated copper nanoparticles capable of being used for conductive ink
A technology of conductive ink and nano particles, which is applied in transportation and packaging, metal processing equipment, coating, etc., can solve the problems of large silver consumption, low cost, and simple preparation process, and achieve green and mild reaction conditions and strong anti-oxidation Ability, high effect of monodispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment is used for the preparation method of the silver-coated copper nanoparticles of conductive ink, comprises the following steps:
[0040] (1) Prepare solution a; add 0.25 g of copper nitrate trihydrate and 0.50 g of polyvinylpyrrolidone into 100 ml of ethylene glycol, stir for 5 minutes and then heat to 100° C. to obtain solution a;
[0041] (2) Prepare solution b; add 3.2g ascorbic acid in 200ml ethylene glycol, stir well and keep the same temperature as solution a to obtain solution b;
[0042] (3) Add solution b to solution a, and continue to stir for 30 minutes to obtain a mixed solution; then add 0.12 g of silver nitrate to 10 ml of deionized water, and add the above mixed solution after completely dissolving, react for 20 minutes and cool to room temperature, 8000r / min The deionized water was centrifuged and washed 4 times, and the precipitate was taken and dried at room temperature for 6 hours under the condition of a vacuum degree of less than 0.01...
Embodiment 2
[0045] This embodiment is used for the preparation method of the silver-coated copper nanoparticles of conductive ink, comprises the following steps:
[0046] (1) Prepare solution a; add 0.45 g of copper acetylacetonate and 1.05 g of cetyltrimethylammonium bromide into 140 ml of diethylene glycol, stir for 15 minutes and then heat to 120° C. to obtain solution a;
[0047] (2) Prepare solution b; add 2.5g of ascorbic acid into 80ml of diethylene glycol, stir well and keep the same temperature as solution a to obtain solution b;
[0048] (3) Add solution b to solution a, and continue to stir for 20 minutes; then add 0.36 g of silver nitrate to 15 ml of deionized water, add the above solution after completely dissolving, react for 100 minutes, cool to room temperature, and centrifuge and wash with 6000 r / min deionized water 4 times, the precipitate was taken and dried at room temperature for 12 hours under the condition that the vacuum degree was less than 0.01 MPa to obtain silv...
Embodiment 3
[0051] This embodiment is used for the preparation method of the silver-coated copper nanoparticles of conductive ink, comprises the following steps:
[0052] (1) Prepare solution a; add 0.58g of copper hydroxide and 2.16g of polyethylene glycol-2000 into 80ml of glycerol, stir for 30min and then heat to 150°C to obtain solution a;
[0053] (2) Prepare solution b; add 2.9g of ascorbic acid into 150ml of glycerol, stir well and keep the same temperature as solution a to obtain solution b;
[0054] (3) Add solution b to solution a, and continue to stir for 40 minutes; then add 0.29 g of silver nitrate to 8 ml of deionized water, add the above solution after completely dissolving, react for 240 minutes, cool to room temperature, and centrifuge wash with 3000 r / min deionized water 4 times, the precipitate was taken and dried at room temperature for 10 h under the condition of a vacuum degree of less than 0.01 MPa to obtain silver-coated copper nanoparticles.
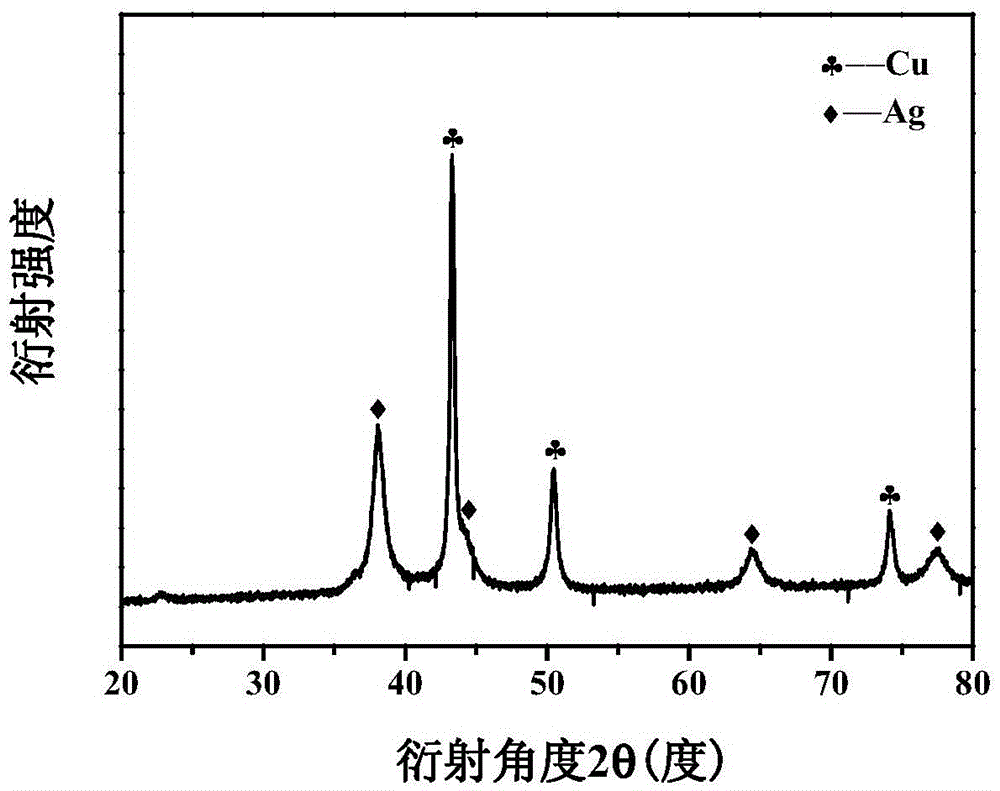
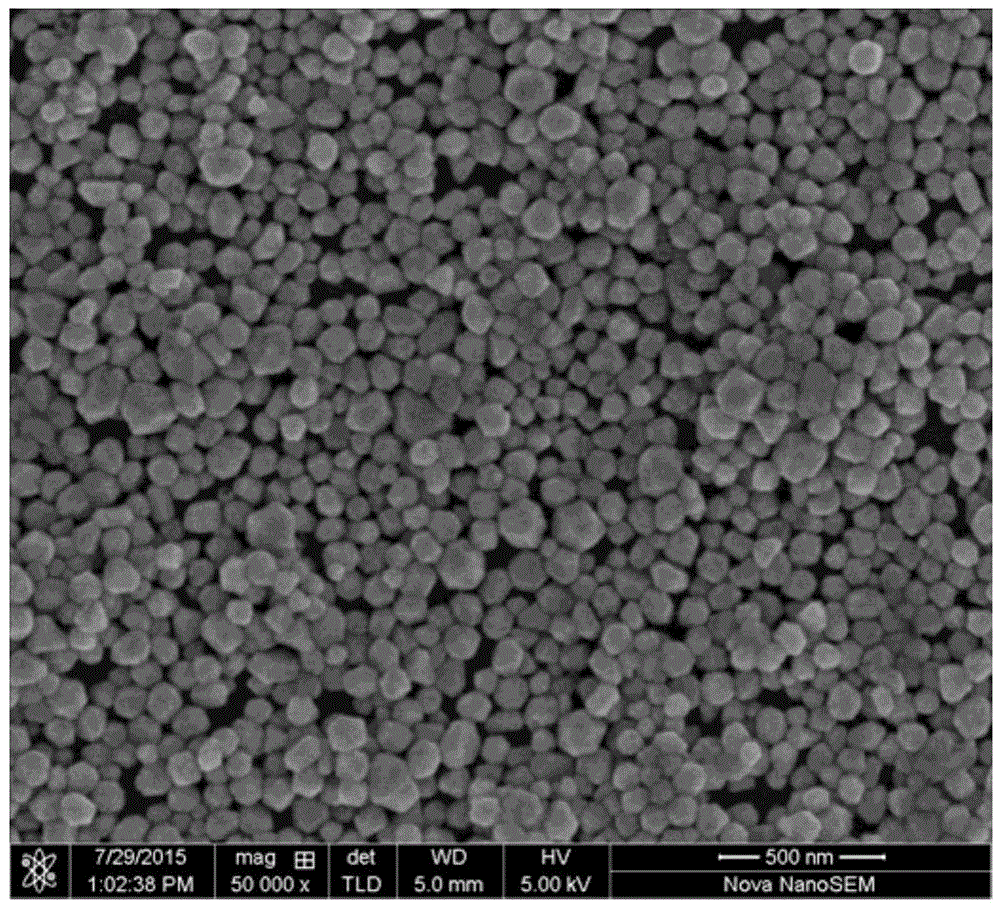
[0055] The XRD patt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com