Crystal form G of ibrutinib and preparation method
A technology of ibrutinib and its crystal form, which is applied in the field of medicinal chemistry, can solve problems such as difficulty in implementing industrialization of stirring, low absorption bioavailability, and poor control of the production process, achieving good controllability and reproducibility, The effect of high bioavailability and cheap solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
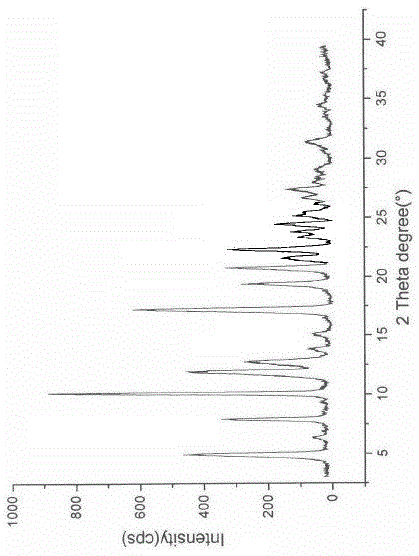
[0019] Add 10 g of ibrutinib amorphous substance into a mixed solution of 100 ml of ethyl acetate and methanol (the volume ratio of isopropanol and methanol is 2:1), heat to reflux to dissolve, stir for 10 hours after dissolving, add 100 ml of water, and stir for 2 Hours, filtered, heated and dried while grinding to obtain crystal G, using Cu-Kα radiation, the X-ray powder diffraction represented by 2θ angle is as follows figure 1 As shown, the HPLC purity was 99.92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com