Trivalent gold complex and application thereof to hydrogen manufacturing through photocatalytic reduction water
A gold complex and chemical technology, applied in the field of photocatalyst, can solve the problems of poor ability to absorb visible light and slow catalytic rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
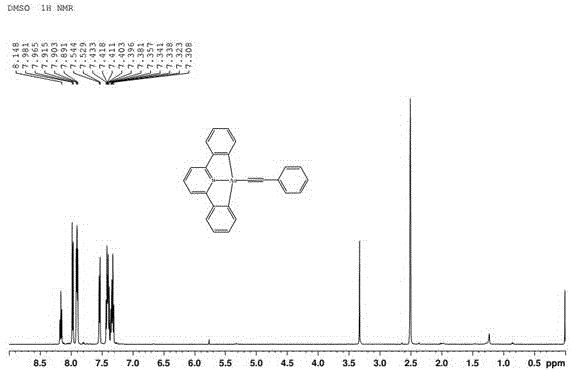
[0032] 0.43mmol[Au(C^N^C)Cl] and 0.1ml phenylacetylene (molar ratio is 1:2) were dissolved in 20mg cuprous iodide, 35mL dichloromethane and 2mL triethylamine molar ratio: 1 :5460:14 in the mixed solution of composition, stirred at room temperature for 6h, after the reaction was finished, the solvent was spin-dried and carried out chromatographic separation in a silica gel column with a volume ratio of 2:1 n-hexane and dichloromethane as the eluent, The trivalent gold complex was obtained through the recrystallization process.
[0033] The trivalent gold complex is carried out nuclear magnetic resonance (H spectrogram sees figure 1 ), measured: 1 HNMR (DMSO, 500MHz): δ8.16 (1H, t, J = 8.0), 7.98 (2H, d, J = 8.0), 7.90 (4H, t, J = 6.0), 7.54 (2H, d, J = 7.3), 7.41(4H,m), 7.33(3H,m). Elemental analysis, calculated value: C 25 h 16 NAu: C, 56.94; H, 3.06; N, 2.66. Calculated: C, 56.66; H, 3.07; N, 2.67. ESI-MS (Methanol): Found: 1076.83 [2×M+Na] + . Calculated value: 527.37. ...
Embodiment 2
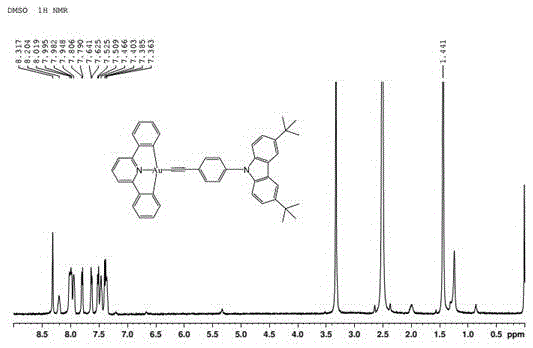
[0035] Dissolve 0.52mmol[Au(C^N^C)Cl] and 0.79ml3,6-di-tert-butyl-9-(4-ethynylphenyl)-9H-carbazole in a molar ratio of 2:3 in In the mixed solution that 20mg cuprous iodide, 35mL dichloromethane and 2mL triethylamine form, stir 8h at room temperature, after the reaction finishes, after the solvent is spin-dried, in the silica gel column, be that the volume ratio is 2:1 n-hexane and Dichloromethane is used as the eluent for chromatographic separation, and the trivalent gold complex is obtained through the recrystallization process.
[0036] The trivalent gold complex is carried out nuclear magnetic resonance (H spectrogram sees figure 2 ), measured: 1 HNMR(DMSO,500MHz):δ8.31(2H,s),8.20(1H,s),7.99(4H,t,J=9.2),7.94(2H,s),7.80(2H,d,J=7.6 ),7.62(2H,d,J=7.9),7.52(2H,d,J=7.9),7.46(2H,s),7.38(4H,m),1.44(16H,s). Elemental analysis, calculated value :C 46 h 39 NAu: C, 68.74; H, 5.02; N, 1.74. Found: C, 68.48; H, 5.14; + . Calculated value: 804.77.
Embodiment 3
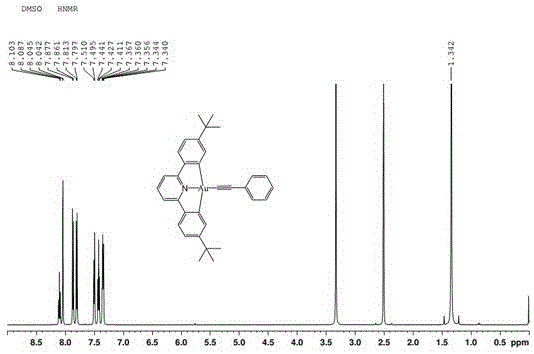
[0038] Mix 0.1mL phenylacetylene with 0.43mmol[Au(C^N^C)Cl], 0.38mmol[Au( t BuC^N^C t Bu)Cl] (molar ratio is 2:5) was added in the mixed solution that is made up of 20mg cuprous iodide, 35mL methylene chloride and 2mL triethylamine, stirred at room temperature for 6h, after the reaction was finished, the solvent was spin-dried Chromatographic separation was carried out on a silica gel column with n-hexane and dichloromethane at a volume ratio of 2:1 as the eluent, and the trivalent gold complex was obtained through recrystallization.
[0039] The trivalent gold complex is carried out nuclear magnetic resonance (H spectrogram sees image 3 ), measured: 1 HNMR (DMSO, 500MHz): δ8.10 (1H, t, J = 8.0), 8.04 (2H, d, J = 1.5), 7.86 (2H, d, J = 8.0), 7.81 (2H, d, J = 8.2), 7.49 (2H, d, J = 7.3), 7.42 (2H, t, J = 7.5), 7.35 (3H, m), 1.34 (18H, s). Elemental analysis, calculated value: C 33 h 32 NAu: C, 61.97; H, 5.04; N, 2.19. Found: C, 61.86; H, 5.12; + . Calculated value: 639.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com