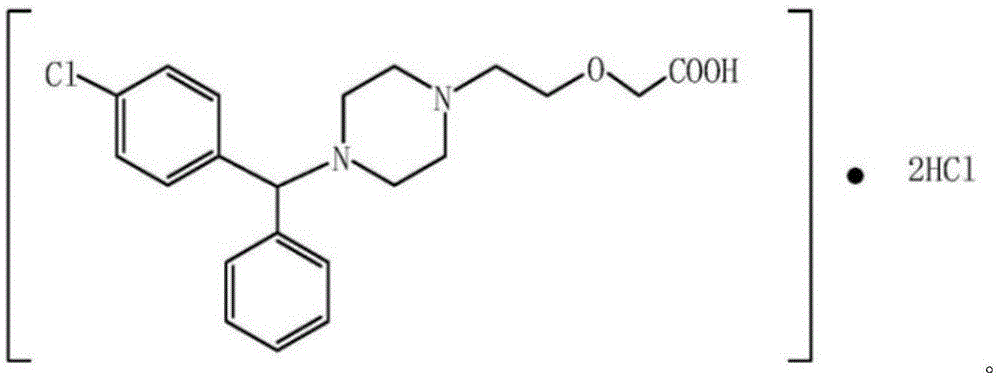
Cetirizine hydrochloride gel as well as preparation method and application thereof
A technology of cetirizine hydrochloride and diazine gel, which is applied in the field of medicine, can solve the problems of affecting bioavailability and reducing the biological activity of cetirizine, and achieve the effects of easy quality, rapid action and fast drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 cetirizine hydrochloride gel
[0035] prescription:
[0036]
[0037] Preparation Process:
[0038] Prepare Carbomer-940 into a solution with a concentration of 4% and let it stand overnight to make it fully swell; take an appropriate amount of purified water, dissolve propylene glycol, add it to the above Carbomer solution and mix evenly, then add triethanolamine while stirring to make Gel-forming matrix A; dissolve cetirizine hydrochloride in a small amount of purified water, add ethanol, slowly add it to A while stirring, and finally add purified water to a sufficient amount, and stir well to obtain.
Embodiment 2
[0039] The preparation of embodiment 2 cetirizine hydrochloride gel
[0040] prescription:
[0041]
[0042]
[0043] Preparation Process:
[0044] Prepare Carbomer-940 into a solution with a concentration of 4% and let it stand overnight to make it fully swell; take an appropriate amount of purified water, dissolve propylene glycol, add it to the above Carbomer solution and mix evenly, then add triethanolamine while stirring to make Gel-forming matrix A; dissolve cetirizine hydrochloride in a small amount of purified water, add ethanol, slowly add it to A while stirring, and finally add purified water to a sufficient amount, and stir well to obtain.
Embodiment 3
[0046] prescription:
[0047]
[0048] Preparation Process:
[0049] Prepare Carbomer-940 into a solution with a concentration of 4% and let it stand overnight to make it fully swell; take an appropriate amount of purified water, dissolve propylene glycol, add it to the above Carbomer solution and mix evenly, then add triethanolamine while stirring to make Gel-forming matrix A; dissolve cetirizine hydrochloride in a small amount of purified water, add ethanol, slowly add it to A while stirring, and finally add purified water to a sufficient amount, and stir well to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com