Sitafloxacin hydrate granules and preparation method of sitafloxacin hydrate granules
A technology of sitafloxacin and granules, which is applied in the field of medicine, can solve the problems of low dissolution rate and low uniformity of drug granules, and achieve the effects of high dissolution rate, rapid dissolution and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
[0023] The prescription of table 1 embodiment 1-4 (unit: g)
[0024]
[0025] Preparation Process:
[0026] According to the prescription amount, sitafloxacin was crushed through a 140 mesh sieve; mannitol was added to silicon dioxide and crushed through a 200 mesh sieve; aspartame was crushed through a 200 mesh sieve; the remaining pharmaceutical excipients were passed through a 140 mesh sieve; Promellose, vanillin essence and other pharmaceutical excipients are mixed evenly, then add sitafloxacin, mannitol, silicon dioxide mixture and mix evenly; spray 30% ethanol solution of hypromellose, granulate, Then add coating solution for coating, then spray 20% aqueous solution of vanillin essence, dry, granulate, sieve out the granules between 40-140 mesh, and pack.
[0027] The preparation method of the coating solution: take Eudragit L30D55 in the formula amount, add 6ml of purified water, triethyl citrate, lactose, talcum powder, stir, and make the mixture evenly.
[0028...
Embodiment 5
[0037] The preparation of sitafloxacin granule composition: it comprises the following steps: (1) preparation concentration is the carrageenan solution of 5%: get carrageenan 10g, add 200m1 hot water and make dissolving, cool to room temperature, use 0.1mol / L Sodium hydroxide solution to adjust the pH to 5.5, for later use; weigh 105g of sitafloxacin, add carrageenan solution to make it wet and repeat the granulation in a swinging granulator, dry for 4 hours, and pulverize to make sitafloxacin Carrageenan granule; (2) preparation concentration is 5% sodium chloride solution 25ml, adjusts pH to be 8.5 with 0.1mol / L sodium hydroxide solution, for subsequent use; The sitafloxacin carrageenan granule in step (1) Add sodium chloride solution to make it wet and solidify, dry at 65°C for 4 hours, and prepare sitafloxacin inclusion complex; (3) Weigh 2kg sucrose, 200g starch, and 15g sodium carboxymethylcellulose, and mix them in a wet mixing method. Dry mix in the granulator for 500-...
Embodiment 6
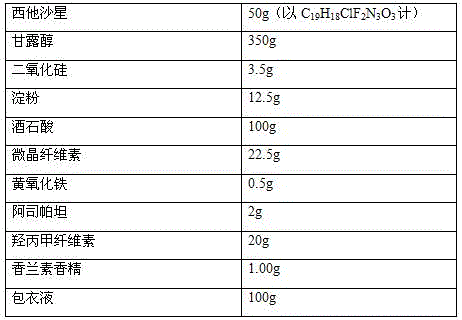
[0039] The prescription of sitafloxacin granules is as follows:
[0040]
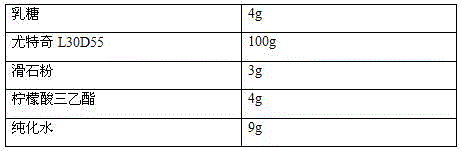
[0041] The coating prescription is as follows:
[0042]
[0043] Preparation Process:
[0044] According to the prescription quantity, each material is pulverized and passed through a mesh sieve, mixed uniformly, granulated, then added with a coating solution for coating, granulated, and packaged to obtain the product. The preparation method of the coating solution: take Eudragit L30D55 in the formula amount, add purified water, plasticizer, porogen, and anti-sticking agent, stir, and mix evenly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com